Alberta NINT/18 June 2008
From 2008.igem.org
(Difference between revisions)
| (4 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Team:Alberta_NINT/Notebook | < Back to notebook]] | ||
| + | |||
| + | [[Alberta_NINT/17_June_2008 | < Previous entry]] | [[Alberta_NINT/19_June_2008 | Next entry >]] | ||
| + | |||
| + | |||
lab work (SD): | lab work (SD): | ||
Results: K102000 + I732018.1 - 134 colonies | Results: K102000 + I732018.1 - 134 colonies | ||
| Line 17: | Line 22: | ||
Ran on 2% agarose gel and cut out desired band | Ran on 2% agarose gel and cut out desired band | ||
Gel purified K102005/B+N | Gel purified K102005/B+N | ||
| - | Ligated our freshly cut K102005/B+N to both our old TA0 that was heated at 50C for 10 minutes and our newly cut TA0 heated at 50C for 10 minutes | + | Ligated our freshly cut K102005/B+N to both our old TA0 that was heated at 50C for 10 minutes and |
| + | our newly cut TA0 heated at 50C for 10 minutes | ||
Transformed XL1-B cells with these new K102007s | Transformed XL1-B cells with these new K102007s | ||
Plated K102007 cells | Plated K102007 cells | ||
| - | Counted number of colonies on K102003 plates from yesterday: 5 on K102003(1) and 2 on K102003(2) and 2 on negative plate | + | Counted number of colonies on K102003 plates from yesterday: 5 on K102003(1) and 2 on K102003(2) |
| + | and 2 on negative plate | ||
Created 7 O/N of these K102003 colonies | Created 7 O/N of these K102003 colonies | ||
| + | [[Image:annotated3.jpg]] | ||
Latest revision as of 22:51, 11 October 2008
< Previous entry | Next entry >
lab work (SD):
Results: K102000 + I732018.1 - 134 colonies
K102000 + I732018.2 - 125 colonies
K102000 (negative control) - 10 colonies
Based on sequencing results, 6 colonies from K102000 + I732018.2 plate were inoculated into LB +
amp50 culture tubes and incubated at 37 C, shaking, overnight.
10 ul of K102009 overnight tube from 05/06/08 was inoculated into LB + amp50 culture tube and
incubated at 37 C, shaking, overnight to create fresh cultures for making a glycerol stock.
For Wayne: Extracted K102011/H+X from gel using QIA quick gel extraction protocol and a min-elute
spin column. DNA was eluted in 15ul Buffer EB.
lab work (JD):
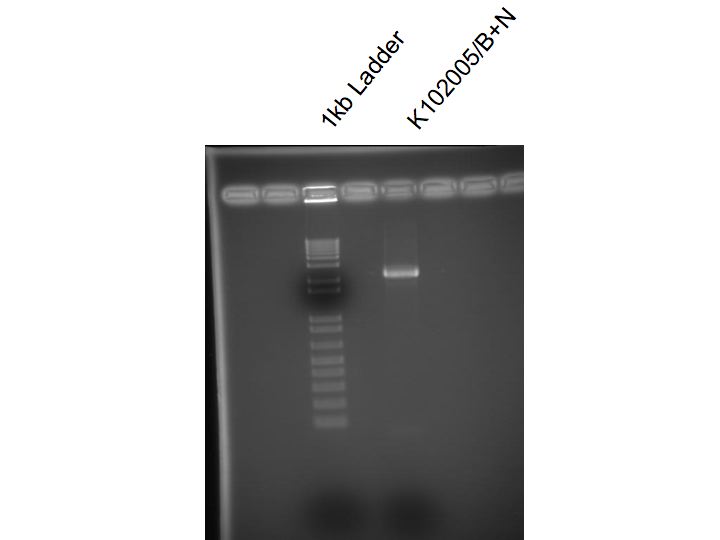
Miniprep of K102005 from O/N
Digested K102005 with BamH1 and Nco1
Ran on 2% agarose gel and cut out desired band
Gel purified K102005/B+N
Ligated our freshly cut K102005/B+N to both our old TA0 that was heated at 50C for 10 minutes and
our newly cut TA0 heated at 50C for 10 minutes
Transformed XL1-B cells with these new K102007s
Plated K102007 cells
Counted number of colonies on K102003 plates from yesterday: 5 on K102003(1) and 2 on K102003(2)
and 2 on negative plate
Created 7 O/N of these K102003 colonies
 "
"