Team:Heidelberg/Notebook/Sensing Group/Notebook/1stweek
From 2008.igem.org
(Difference between revisions)
Chenchenzhu (Talk | contribs) (→Friday, 08/08/2008) |
|||
| (5 intermediate revisions not shown) | |||
| Line 469: | Line 469: | ||
=== Preparations === | === Preparations === | ||
Preparations were started at the end of the week before | Preparations were started at the end of the week before | ||
| - | * Transformation of pDK48 and pTrc99alpha plamids in ''E. coli'' strain | + | * Transformation of pDK48 and pTrc99alpha plamids in ''E. coli'' strain DH5α (0.5 µl plasmid-DNA + 50 µl competent cells) |
* Glycerol-stock of ''Vibrio harveyi'' BB120, BB886, mm30, BB178, BB125 | * Glycerol-stock of ''Vibrio harveyi'' BB120, BB886, mm30, BB178, BB125 | ||
| - | * pick | + | * pick pTrc99α and pDK48 colonies and then incubated in 3 ml LB at 37 overnight |
* ''V. harveyi'' cultured on LB plate | * ''V. harveyi'' cultured on LB plate | ||
| - | * MiniPrep of | + | * MiniPrep of pTrc99α and pDK48 from DH5α |
== Tuesday, 08/05/2008 == | == Tuesday, 08/05/2008 == | ||
=== Cloning of LuxP === | === Cloning of LuxP === | ||
[[Image:HD_080805-LuxP_PCR.png|thumb|150px|LuxP colony PCR]] | [[Image:HD_080805-LuxP_PCR.png|thumb|150px|LuxP colony PCR]] | ||
| - | <div style="float: right; clear:none">[[Image:HD 080805-LuxQ S PCR.png|right|thumb|200px|LuxQ, LuxS PCR]]</div> | + | <div style="float: right; clear:none;">[[Image:HD 080805-LuxQ S PCR.png|right|thumb|200px|LuxQ, LuxS PCR]]</div> |
| + | |||
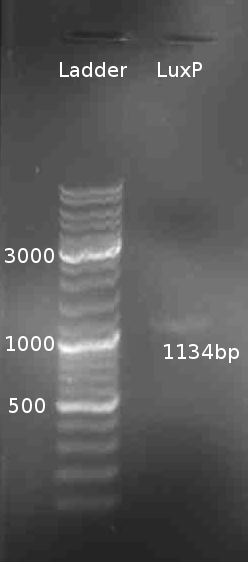
* PCR of LuxP from ''V. harveyi'' colony with the primers LuxPc and LuxPd with Taq polymerase Mastermix. 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) | * PCR of LuxP from ''V. harveyi'' colony with the primers LuxPc and LuxPd with Taq polymerase Mastermix. 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) | ||
* Gel Purification of LuxP eluted in 30 µl H<sub>2</sub>O | * Gel Purification of LuxP eluted in 30 µl H<sub>2</sub>O | ||
| Line 486: | Line 487: | ||
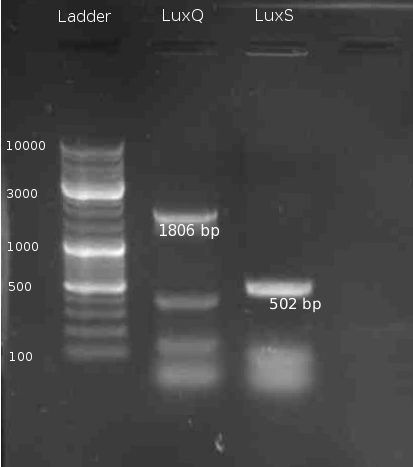
=== Cloning of LuxS === | === Cloning of LuxS === | ||
* PCR of ''V. harveyi'' genome with primers LuxSa and LuxSb and PCR purification 5min @ 95 °C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) with Tag polymerase Mastermix | * PCR of ''V. harveyi'' genome with primers LuxSa and LuxSb and PCR purification 5min @ 95 °C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) with Tag polymerase Mastermix | ||
| - | * digestion of LuxS and | + | * digestion of LuxS and pTrc99α with BamHI/NcoI (NEBuffer 3 + BSA) |
* Ligation with vector:insert ratio 2µl:6µl and 5µl:5µl | * Ligation with vector:insert ratio 2µl:6µl and 5µl:5µl | ||
| Line 511: | Line 512: | ||
== Friday, 08/08/2008 == | == Friday, 08/08/2008 == | ||
* Miniprep of overnight-cultures of the picked colonies Q#6-15 | * Miniprep of overnight-cultures of the picked colonies Q#6-15 | ||
| - | * In silico cloning of LuxQ, S in | + | * In silico cloning of LuxQ, S in pTrc99α and LuxP in pDK48 |
* Test-Digestion of LuxQ with XbaI --> positive result for Q3, 7, 9, 11, 14 | * Test-Digestion of LuxQ with XbaI --> positive result for Q3, 7, 9, 11, 14 | ||
[[Team:Heidelberg/Notebook/Sensing_Group/Notebook/2ndweek | Go to 2nd week]] | [[Team:Heidelberg/Notebook/Sensing_Group/Notebook/2ndweek | Go to 2nd week]] | ||
Latest revision as of 14:01, 29 October 2008


Contents |
Monday, 08/04/2008
Preparations
Preparations were started at the end of the week before
- Transformation of pDK48 and pTrc99alpha plamids in E. coli strain DH5α (0.5 µl plasmid-DNA + 50 µl competent cells)
- Glycerol-stock of Vibrio harveyi BB120, BB886, mm30, BB178, BB125
- pick pTrc99α and pDK48 colonies and then incubated in 3 ml LB at 37 overnight
- V. harveyi cultured on LB plate
- MiniPrep of pTrc99α and pDK48 from DH5α
Tuesday, 08/05/2008
Cloning of LuxP
- PCR of LuxP from V. harveyi colony with the primers LuxPc and LuxPd with Taq polymerase Mastermix. 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles)
- Gel Purification of LuxP eluted in 30 µl H2O
- Digestion of LuxP with SalI and NotI of pDK48 and LuxP-Gel-Purification product: 2 µl NEB-Buffer 3 + 0.5 µl SalI & NotI + 10 µl DNA + 6 µl H2O
- Ligation with vector:insert ratio 2µl:6µl and 5µl:5µl
Cloning of LuxS
- PCR of V. harveyi genome with primers LuxSa and LuxSb and PCR purification 5min @ 95 °C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles) with Tag polymerase Mastermix
- digestion of LuxS and pTrc99α with BamHI/NcoI (NEBuffer 3 + BSA)
- Ligation with vector:insert ratio 2µl:6µl and 5µl:5µl
Wednesday, 08/06/2008
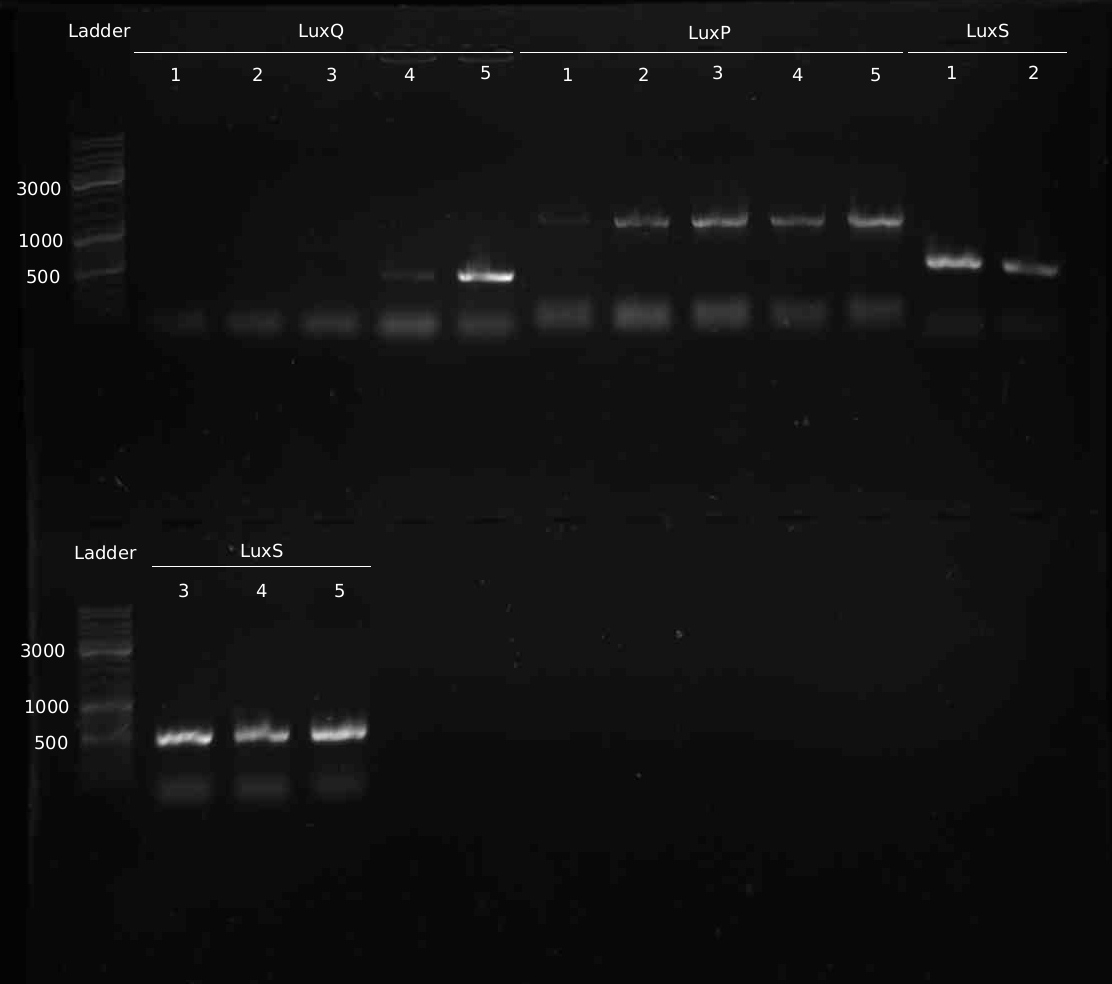
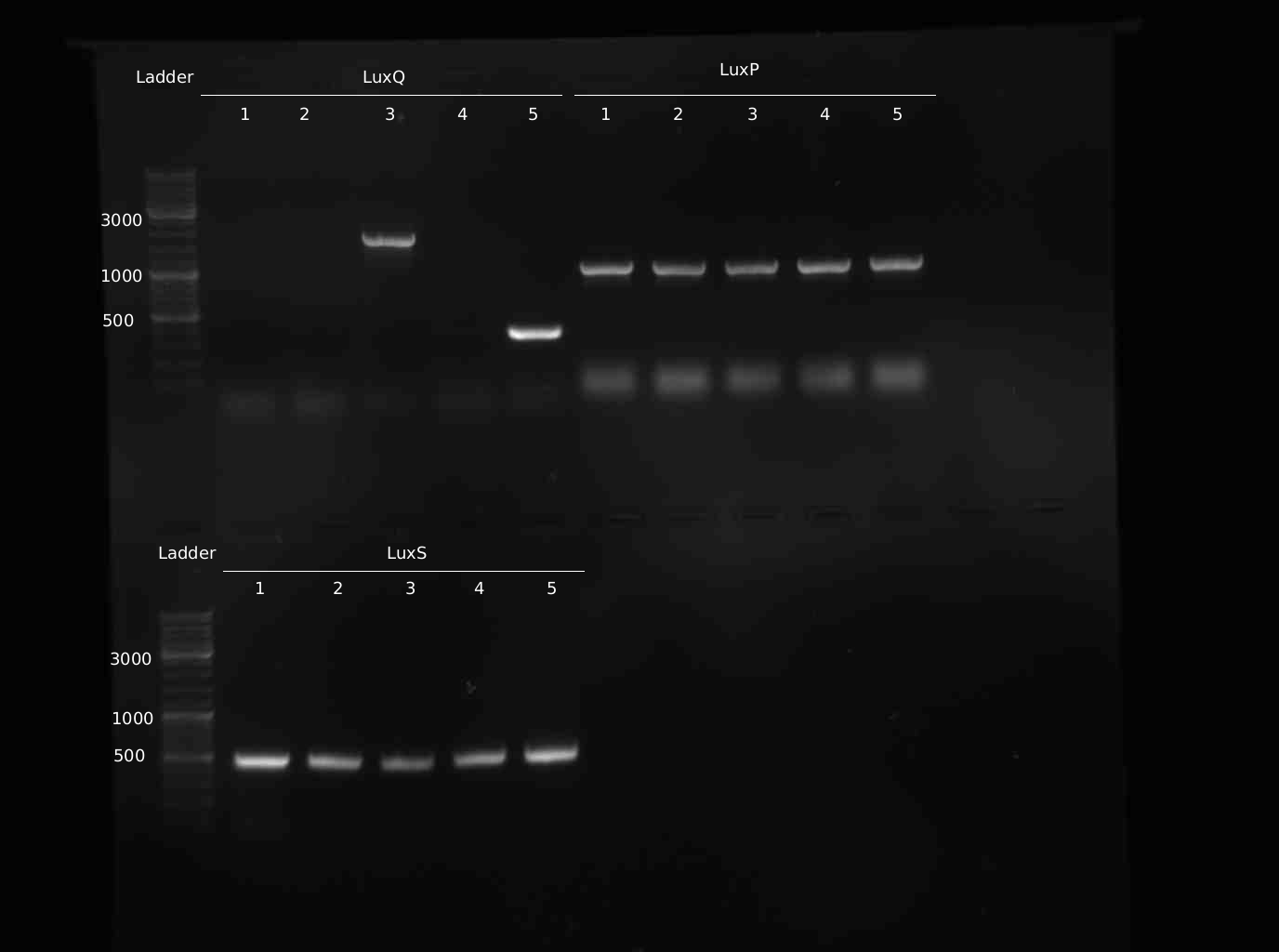
- 5 colonies of LuxQ,S,P transformation (1:1 ligation) picked and analyes with colony-PCR
- Colony-PCR to check for insert. 5min @ 95°C || 30s @ 95°C | 30s @ 58 °C | 2min @ 72°C || 10min @ 72°C | 4°C hold (30 cycles)
- preparation of O/N cultures for Miniprep
Thursday, 08/07/2008
- Miniprep of samples Q#1-5, S#1-5, P#1-5
- PCR from Minipreps to confirm inserts. Same programme as yesterday
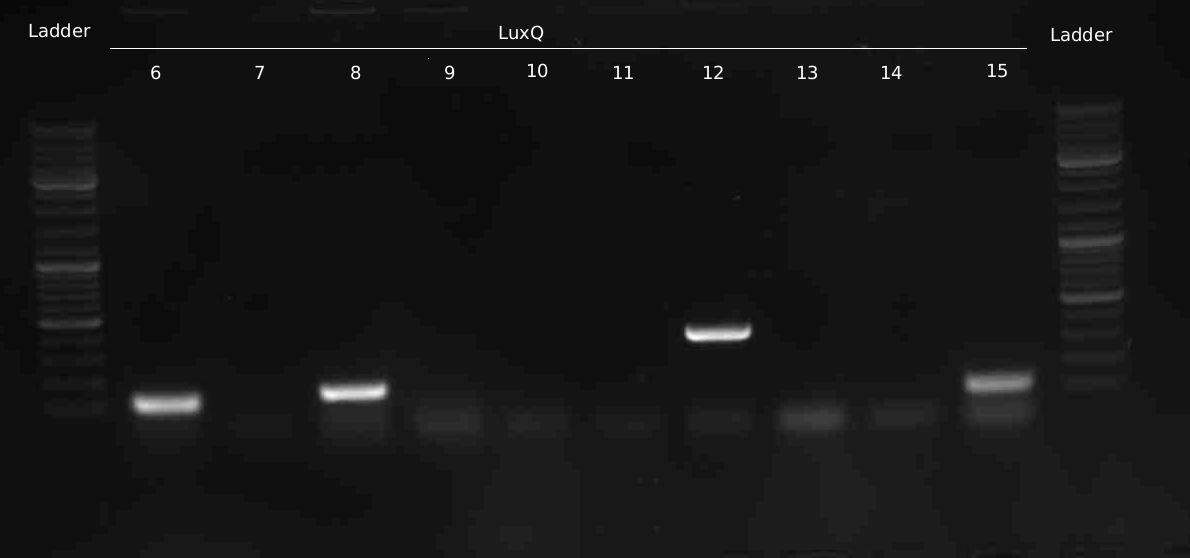
- 10 new colonies of LuxQ-plate were picked and checked via colony-PCR
- Sequencing of LuxS,P,Q positive clones at GATC
Sequencing Results LuxS: correct sequence LuxP: all clones contain mutations, cloning has to be repeated LuxQ: no insert in the picked colonies
Friday, 08/08/2008
- Miniprep of overnight-cultures of the picked colonies Q#6-15
- In silico cloning of LuxQ, S in pTrc99α and LuxP in pDK48
- Test-Digestion of LuxQ with XbaI --> positive result for Q3, 7, 9, 11, 14
 "
"