Team:PennState/diauxie/progress
From 2008.igem.org
| (12 intermediate revisions not shown) | |||
| Line 181: | Line 181: | ||
<hr /> | <hr /> | ||
| - | <p class="start"> | + | <p class="start">Each test construct (promoter + GFP) was cloned into the pSB1A2 plasmid and transformed into several <em>E. coli</em> strains: DH5α, W3110 ∆xylB-G, and W3110 ∆xylB-R. Preliminary induction studies were run to find the optimal induction time and to analyze the linear range for OD versus fluorescence.</p> |
<h6>Test Construct</h6> | <h6>Test Construct</h6> | ||
<img src="https://static.igem.org/mediawiki/2008/1/1b/Test_construct.JPG" alt="[Test Consturct]" title="" style="width: 100%; border: solid 1px #000" /> | <img src="https://static.igem.org/mediawiki/2008/1/1b/Test_construct.JPG" alt="[Test Consturct]" title="" style="width: 100%; border: solid 1px #000" /> | ||
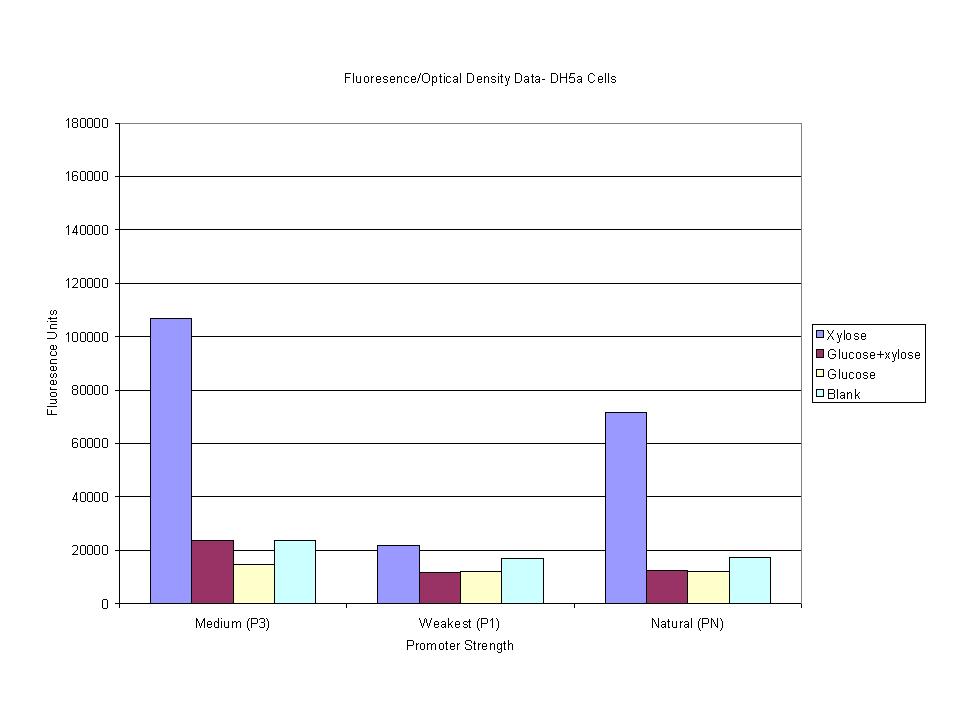
| - | < | + | <p>Tests were then run to compare the levels of induction with various mixes of xylose, glucose and xylose+glucose. The goal was to obtain a noticeably higher level of induction with the xylose/glucose mixture when compared to the wild-type construct.</p> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </td> | |
| + | <table> <!-- this table separates content into column-like quadrants --> | ||
| + | <tr> | ||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="45%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 232: | Line 200: | ||
<div class="gallerytext"><p>DH5a fluoresence</p></div> | <div class="gallerytext"><p>DH5a fluoresence</p></div> | ||
</div> | </div> | ||
| - | + | ||
| + | </td> | ||
| + | |||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="45%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 239: | Line 211: | ||
<div class="gallerytext"><p>W3110 fluoresence</p></div> | <div class="gallerytext"><p>W3110 fluoresence</p></div> | ||
</div> | </div> | ||
| + | |||
| + | </p></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
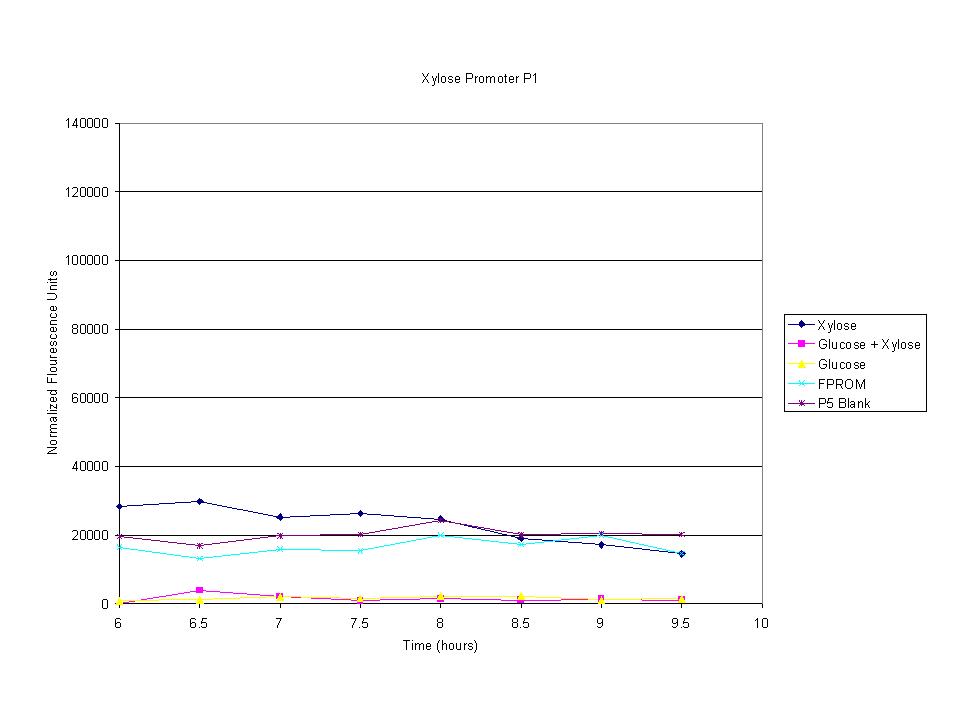
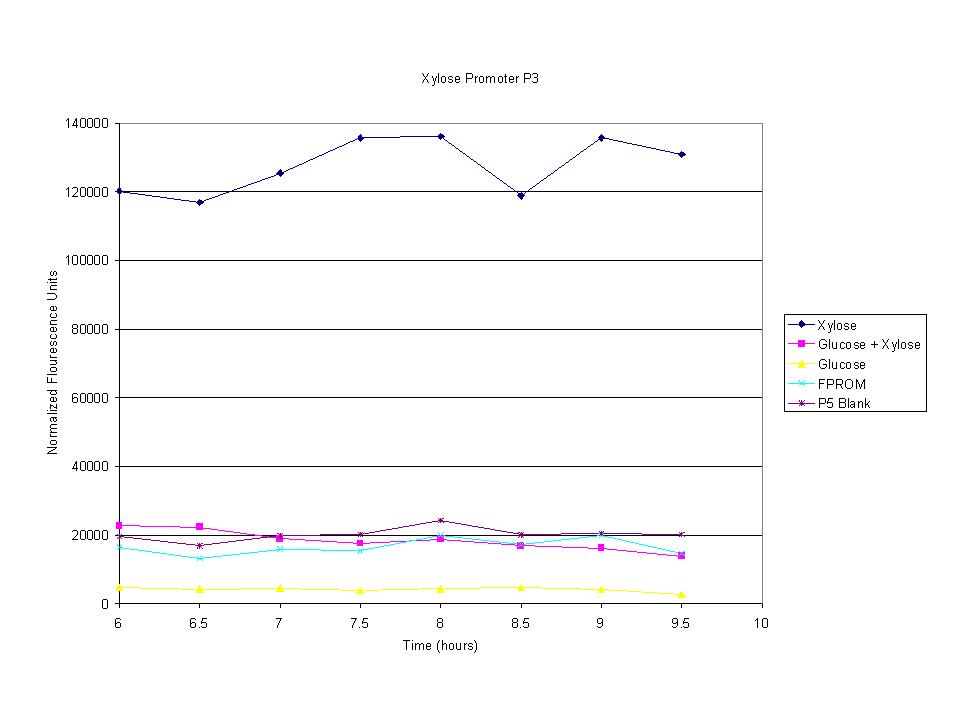
| + | <p>These graphs show the normalized fluoresence strength for PN, P1, and P3 xylose promoters induced with xylose, glucose, and a mixture. The W3110 cells have <em>xylE</em> and <em>xylG</em> knocked out while DH5α still contain the natural xylose transport and metabolim. This data shows that there is little effect on the fluorescence intensity using strains with <em>xylE</em> and <em>xylG</em> sequences deleted. Our next step is to transform these promoters into <em>E. coli</em> cells with deleted xylose metabolism and transporters. </p> | ||
| + | |||
| + | <table> <!-- this table separates content into column-like quadrants --> | ||
| + | <tr> | ||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="33%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 247: | Line 230: | ||
</div> | </div> | ||
| + | |||
| + | </td> | ||
| + | |||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="33%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 254: | Line 242: | ||
</div> | </div> | ||
| + | </td> | ||
| + | |||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="33%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 260: | Line 252: | ||
<div class="gallerytext"><p>P3 induction time</p></div> | <div class="gallerytext"><p>P3 induction time</p></div> | ||
</div> | </div> | ||
| + | </td> | ||
| + | </p></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | <p>The cells were induced iduced with sugars and then allowed to grow with sample being removed every half hour. The goal was to find the induction time where fluorescence starts to level off. The intensity has leveled off and begins to drop after 7.5 hours so this was the growth time for all future tests. | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | <table> <!-- this table separates content into column-like quadrants --> | ||
| + | <tr> | ||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="33%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 268: | Line 274: | ||
</div> | </div> | ||
| + | |||
| + | </td> | ||
| + | |||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="33%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 274: | Line 285: | ||
<div class="gallerytext"><p>P1 linear range</p></div> | <div class="gallerytext"><p>P1 linear range</p></div> | ||
</div> | </div> | ||
| + | </td> | ||
| + | <td style="padding-top:30px; padding-right:30px" valign="top" width="33%"><h4></h4> | ||
| + | <hr /> | ||
</div> | </div> | ||
<div id="psugallery" style="float: right; clear: right; width: 500px;"> | <div id="psugallery" style="float: right; clear: right; width: 500px;"> | ||
| Line 281: | Line 295: | ||
<div class="gallerytext"><p>P3 linear range</p></div> | <div class="gallerytext"><p>P3 linear range</p></div> | ||
</div> | </div> | ||
| + | </td> | ||
| + | |||
| + | </p></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
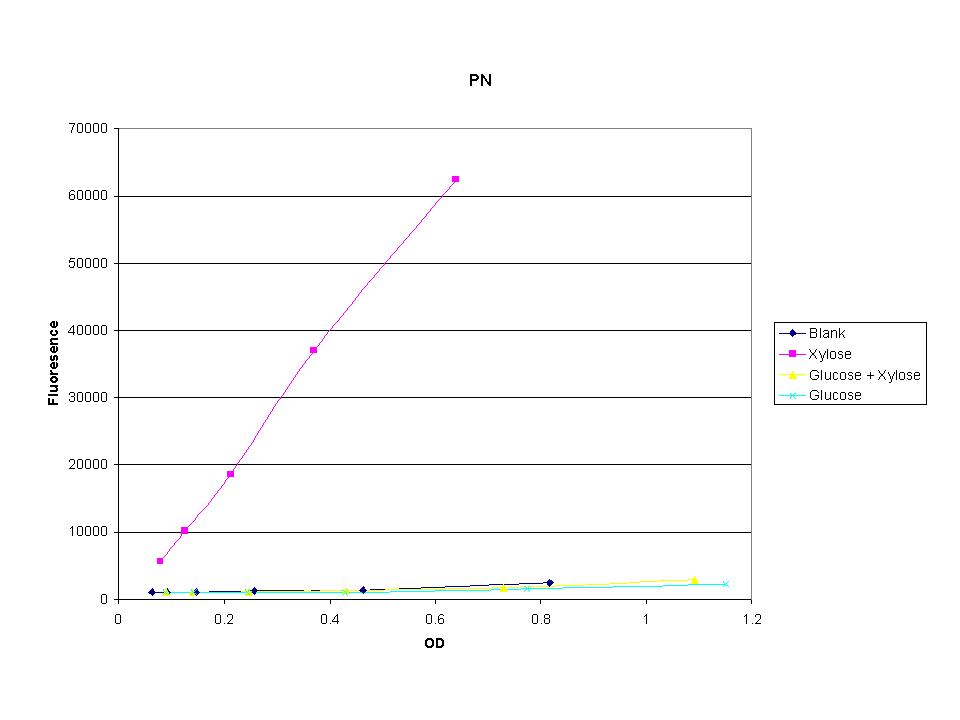
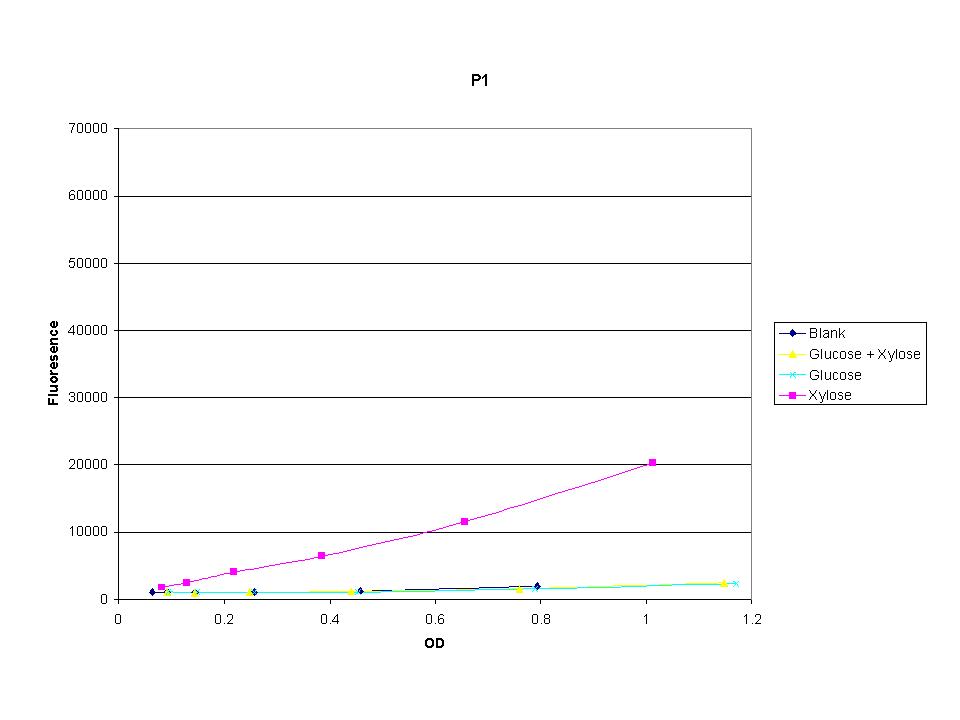
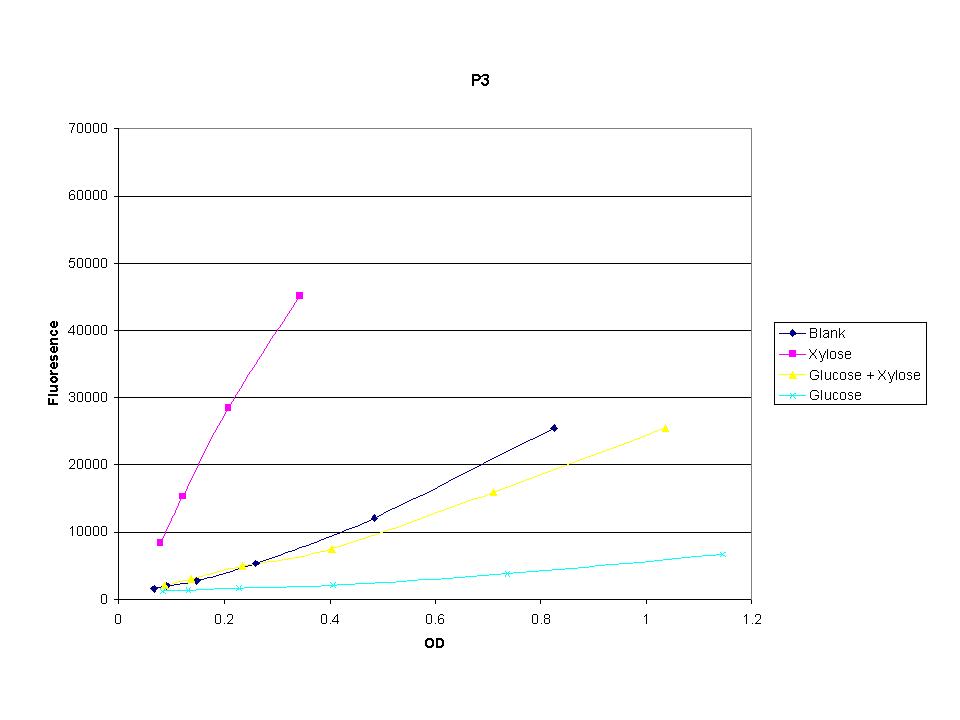
| + | <p>The three promoters analyzed show linear behavior in the optical density range. The strength of fluorescence did change depending on the promoter. With this information we were able to accurately normalize fluorescence data. | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Latest revision as of 02:19, 30 October 2008

| Home | The Team | The Project | Parts | Notebook |
Diauxie EliminationNHR Biosensors
|
Progress & Results
Each test construct (promoter + GFP) was cloned into the pSB1A2 plasmid and transformed into several E. coli strains: DH5α, W3110 ∆xylB-G, and W3110 ∆xylB-R. Preliminary induction studies were run to find the optimal induction time and to analyze the linear range for OD versus fluorescence. Test Construct![[Test Consturct]](https://static.igem.org/mediawiki/2008/1/1b/Test_construct.JPG)
Tests were then run to compare the levels of induction with various mixes of xylose, glucose and xylose+glucose. The goal was to obtain a noticeably higher level of induction with the xylose/glucose mixture when compared to the wild-type construct. |
|
|
|
These graphs show the normalized fluoresence strength for PN, P1, and P3 xylose promoters induced with xylose, glucose, and a mixture. The W3110 cells have xylE and xylG knocked out while DH5α still contain the natural xylose transport and metabolim. This data shows that there is little effect on the fluorescence intensity using strains with xylE and xylG sequences deleted. Our next step is to transform these promoters into E. coli cells with deleted xylose metabolism and transporters.
|
|
|
|
The cells were induced iduced with sugars and then allowed to grow with sample being removed every half hour. The goal was to find the induction time where fluorescence starts to level off. The intensity has leveled off and begins to drop after 7.5 hours so this was the growth time for all future tests.
|
|
|
|
The three promoters analyzed show linear behavior in the optical density range. The strength of fluorescence did change depending on the promoter. With this information we were able to accurately normalize fluorescence data.
 "
"