Team:Chiba/Calendar-Home/16 October 2008
From 2008.igem.org
(Difference between revisions)
(→Team:Demo-Rs) |
(→Laboratory work) |
||
| (2 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
[[Team:Chiba/Calendar-Home/15 October 2008|15 October 2008 <]]|[[Team:Chiba/Calendar-Home/17 October 2008|> 17 October 2008]] | [[Team:Chiba/Calendar-Home/15 October 2008|15 October 2008 <]]|[[Team:Chiba/Calendar-Home/17 October 2008|> 17 October 2008]] | ||
| + | |||
| + | ==Laboratory work== | ||
| + | === Team:Demo-Is === | ||
| + | #Pre-culture | ||
| + | ##Picked and cultured the following glycerol stocks in 2mL of LB: | ||
| + | ###LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (JW1908) | ||
| + | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), (XL10G) | ||
| + | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]), (XL10G) | ||
| + | ##Cultured at 37°C for 12h. | ||
| + | #Culture | ||
| + | ##Added 6.25% each of the pre-cultures to new LB medium. | ||
| + | ###LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] | ||
| + | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]) | ||
| + | ##Cultured at 37°C for 4~5h | ||
| + | #Wash | ||
| + | ##Transfer 10mL each of the culture to 50mL centrifuge tubes. | ||
| + | ##Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant. | ||
| + | ##Added LB-Amp to each centrifuge tube: | ||
| + | ###10mL to the tube that contains [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] | ||
| + | ###5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | ||
| + | ##Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant. | ||
| + | ##10mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | ||
| + | ##Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant. | ||
| + | ##5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | ||
| + | #Mix | ||
| + | ##Mixed the sender cells [http://partsregistry.org/Part:BBa_K084012 BBa_K084012] and [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] both with [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] at a 1:1 ratio. | ||
| + | ##Added 100μL each to a 96-well shallow plate (as shown in the figure). | ||
| + | ###Green part is [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1 | ||
| + | ###Red part is [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1 | ||
| + | ###Uncolored part is [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] alone. | ||
| + | #Culture and observe results | ||
| + | |||
| + | ====Results==== | ||
| + | [[Image:Team-Chiba-demo-mihon.gif|200px]] Green region: sender=LuxI, | ||
| + | Red circular region: sender=Las I. | ||
| + | |||
| + | <gallery> | ||
| + | Image:Team-Chiba-demo-1.JPG|0 h | ||
| + | Image:Team-Chiba-demo-2.JPG|4 h <br>(Lux I GFP detected) | ||
| + | Image:Team-Chiba-demo-3.JPG|8 h <br>(Lux I GFP and Las I GFP detected) | ||
| + | </gallery> | ||
| + | |||
| + | |||
| + | LuxI GFP is detected at 4h following mixing while LasI GFP is detected | ||
| + | after 8h, thus successfully demonstrating time-delay depending on the | ||
| + | sender used. | ||
Latest revision as of 00:50, 30 October 2008
15 October 2008 <|> 17 October 2008
Laboratory work
Team:Demo-Is
- Pre-culture
- Picked and cultured the following glycerol stocks in 2mL of LB:
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (JW1908)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), (XL10G)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]), (XL10G)
- Cultured at 37°C for 12h.
- Picked and cultured the following glycerol stocks in 2mL of LB:
- Culture
- Added 6.25% each of the pre-cultures to new LB medium.
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)])
- Cultured at 37°C for 4~5h
- Added 6.25% each of the pre-cultures to new LB medium.
- Wash
- Transfer 10mL each of the culture to 50mL centrifuge tubes.
- Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant.
- Added LB-Amp to each centrifuge tube:
- 10mL to the tube that contains [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- 5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 10mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Mix
- Mixed the sender cells [http://partsregistry.org/Part:BBa_K084012 BBa_K084012] and [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] both with [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] at a 1:1 ratio.
- Added 100μL each to a 96-well shallow plate (as shown in the figure).
- Green part is [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Red part is [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Uncolored part is [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] alone.
- Culture and observe results
Results
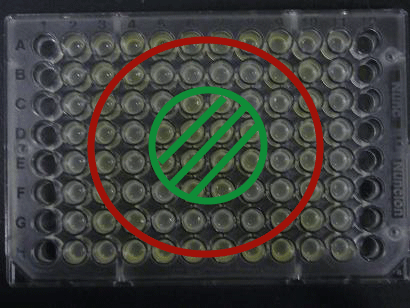 Green region: sender=LuxI,
Red circular region: sender=Las I.
Green region: sender=LuxI,
Red circular region: sender=Las I.
LuxI GFP is detected at 4h following mixing while LasI GFP is detected
after 8h, thus successfully demonstrating time-delay depending on the
sender used.
 "
"