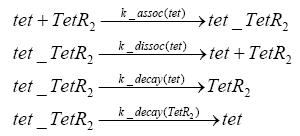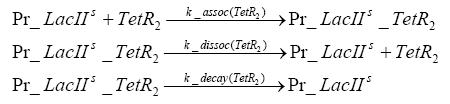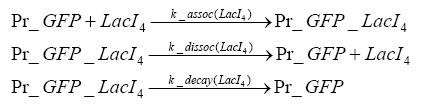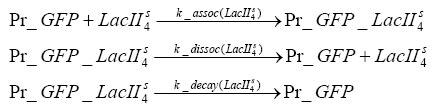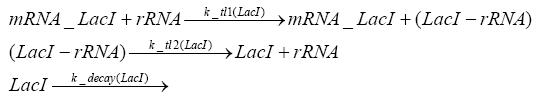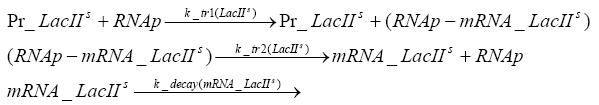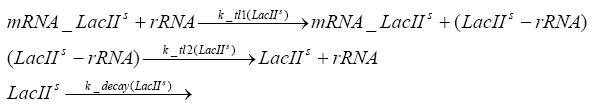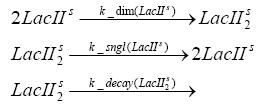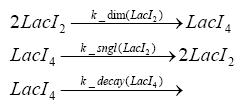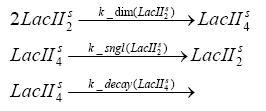Team:ETH Zurich/Modeling/Switch Circuit
From 2008.igem.org
(→Switch Circuit) |
|||
| (37 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
==Switch Circuit== | ==Switch Circuit== | ||
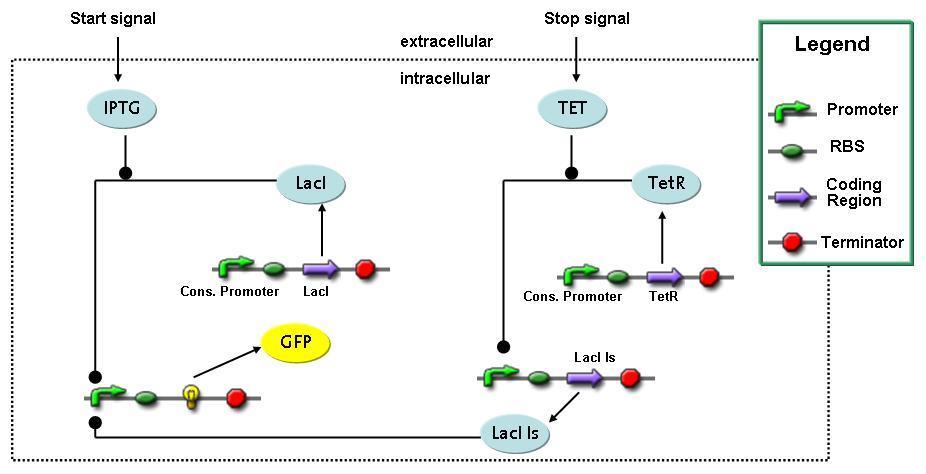
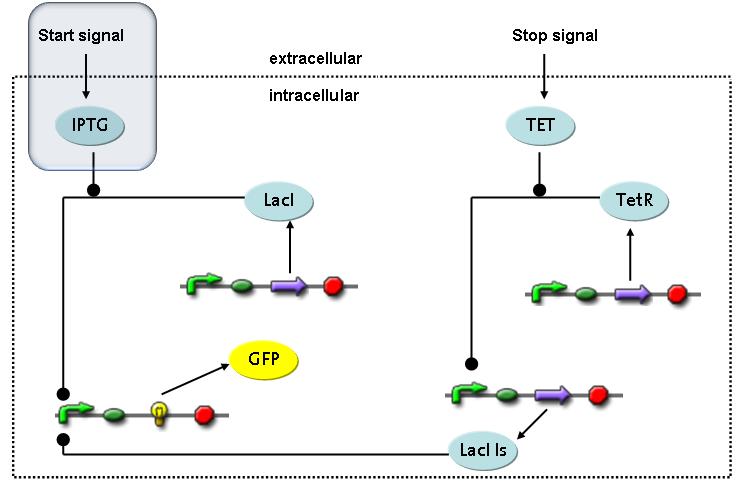
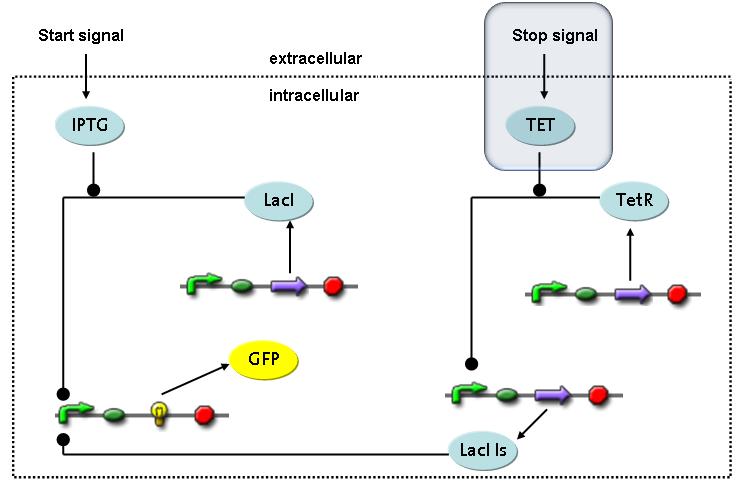
| - | The designed switching circuit is driven by two input signals – a start signal initiates the synthesis of a specific protein and a terminating signal switches gene expression off. The goal of this system is to control expression of | + | The designed switching circuit is driven by two input signals – a start signal initiates the synthesis of a specific protein and a terminating signal switches gene expression off. The goal of this system is to control expression of restriction enzymes in order to delete genome fragments ''in vivo''. |
In order to do some preliminary experiments, the restriction enzyme has been substituted by a fluorescent protein.<br> | In order to do some preliminary experiments, the restriction enzyme has been substituted by a fluorescent protein.<br> | ||
| - | The detailed mechanism is described [[Team:ETH_Zurich/Wetlab/Switch_Circuit#Switch_Circuit| here]]. | + | The detailed mechanism is described [[Team:ETH_Zurich/Wetlab/Switch_Circuit#Switch_Circuit| here]]. It follows a summarized version of it's mode of operation:<br> |
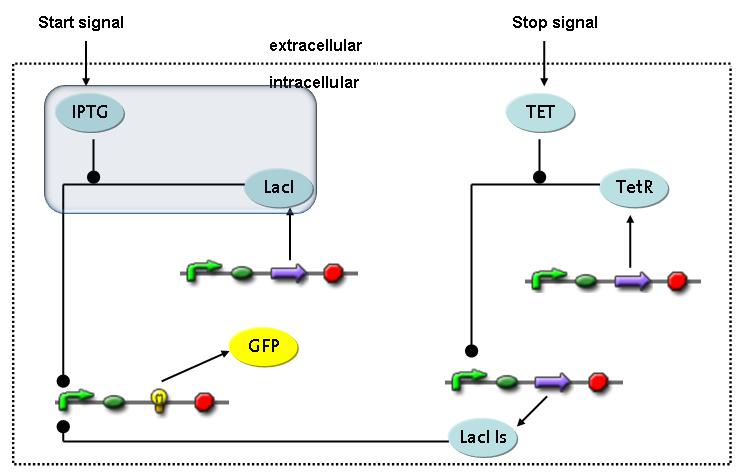
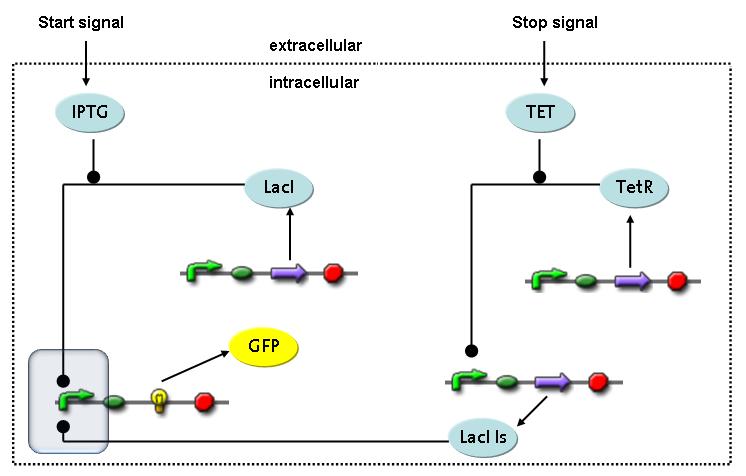
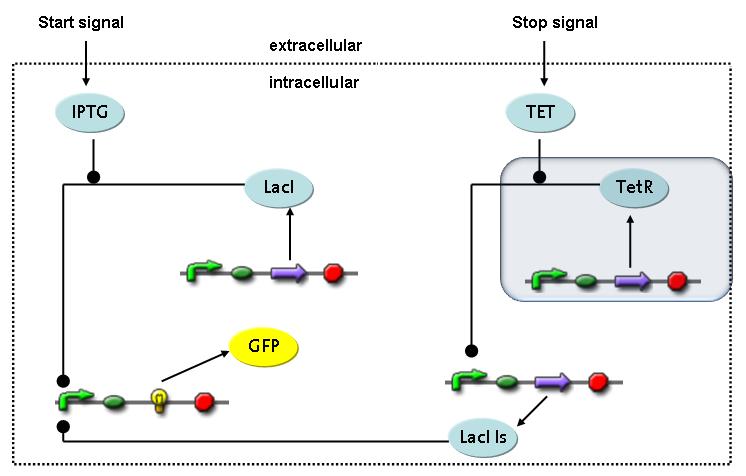
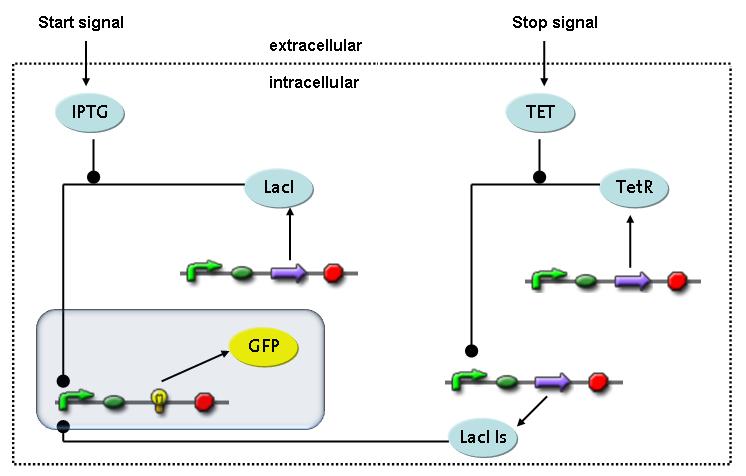
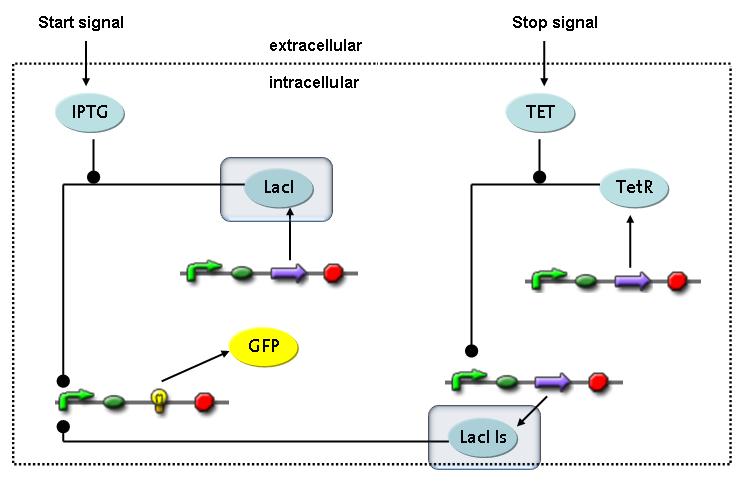
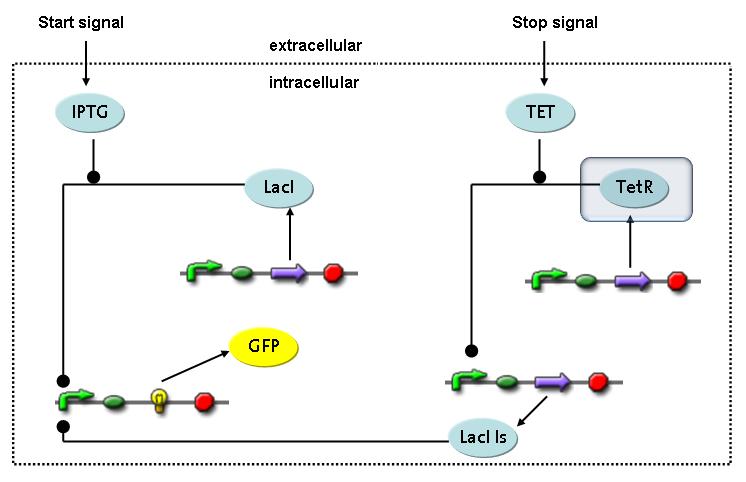
Fluorescent protein gene expression is under control of LacI and can be induced by the addition of IPTG. In order to stop gene expression, the IPTG-sensitive LacI is replaced by IPTG-insensitive LacIIs, which shuts off fluorescent gene expression again. The synthesis of LacIIs is started by the addition of Tetracyclin (tet) to the system, which binds to the tet repressor TetR and thus de-represses the expression of the LacIIs gene. The fluorescent protein is tagged, so that it is degraded faster by the Clp protease and vanishes faster from the system as its expression is stopped.<br> | Fluorescent protein gene expression is under control of LacI and can be induced by the addition of IPTG. In order to stop gene expression, the IPTG-sensitive LacI is replaced by IPTG-insensitive LacIIs, which shuts off fluorescent gene expression again. The synthesis of LacIIs is started by the addition of Tetracyclin (tet) to the system, which binds to the tet repressor TetR and thus de-represses the expression of the LacIIs gene. The fluorescent protein is tagged, so that it is degraded faster by the Clp protease and vanishes faster from the system as its expression is stopped.<br> | ||
| Line 26: | Line 26: | ||
=== Implementation === | === Implementation === | ||
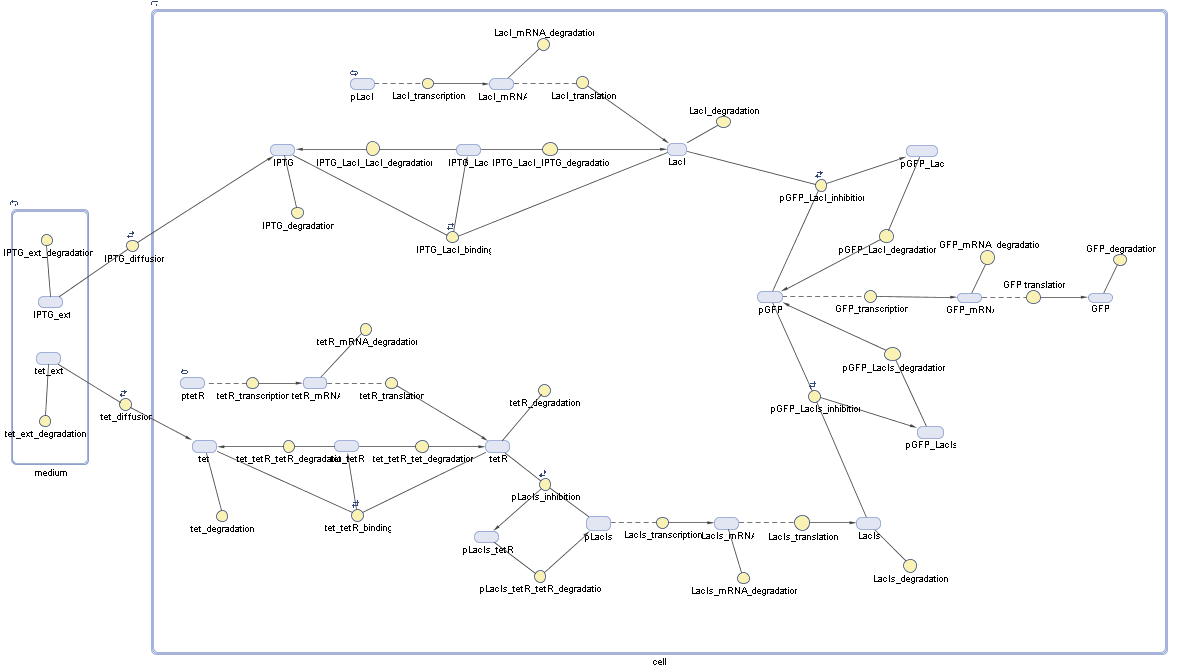
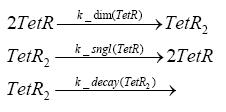
| - | + | In the implementation, some minor parts that do not have significant effects on the aspired results have been neglected for the sake of simplicity. In the MATLAB-model of the different protein-expressions, the RNA-polymerase and the ribosomes have not been taken into account, in the transcription and translation respectively. Furthermore, the effects of the dimerization of TetR as well as the impacts of dimerization and tetramerization of LacI and LacIIs [https://2008.igem.org/Team:ETH_Zurich/Modeling/Switch_Circuit#References (4)] have not been considered in the final implementation. <br> | |
| - | This simplified model still comprises more than 20 different species and over 30 kinetic reactions and was implemented by using the SimBiology Toolbox in MATLAB.<br> | + | This simplified model still comprises more than 20 different molecular species and over 30 kinetic reactions and was implemented by using the SimBiology Toolbox in MATLAB.<br> |
[[Image:model.PNG|thumb|center|900px|diagram view of the model]] | [[Image:model.PNG|thumb|center|900px|diagram view of the model]] | ||
| - | We performed deterministic and stochastic simulations based on Mass | + | We performed deterministic and stochastic simulations based on Mass Action Kinetics. The stochastic simulations turned out to be computationally very exhaustive but generated no further significant information compared to the deterministic simulations.<br> |
=== Simulation Results === | === Simulation Results === | ||
| Line 42: | Line 42: | ||
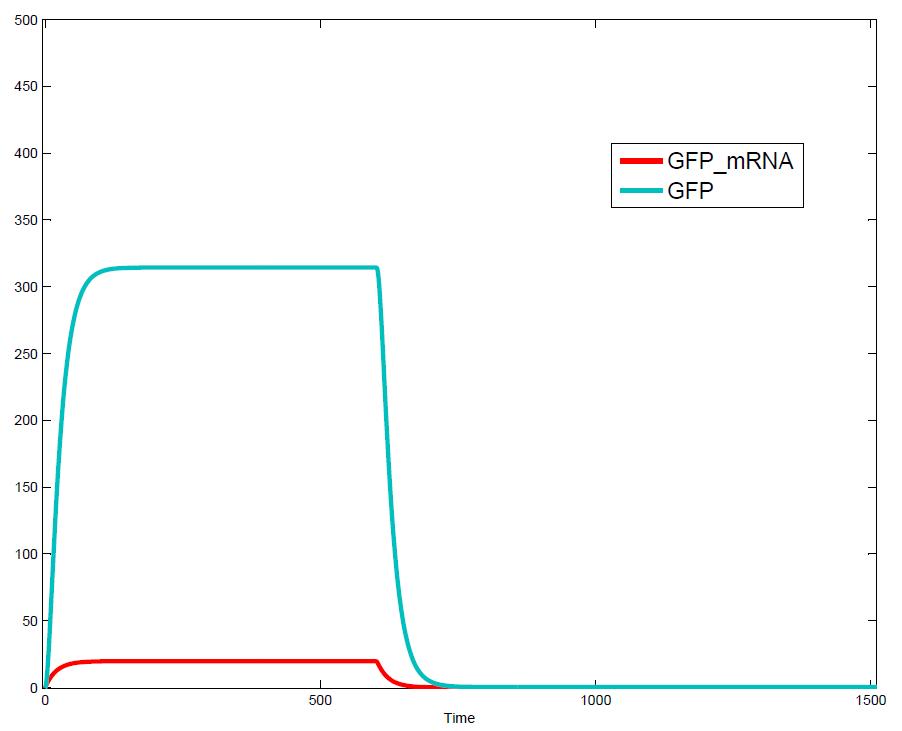
The simulations show that our system actually should create a nice pulse-shaped expression of the fluorescent protein (GFP). This expression can be initiated by inducing with IPTG and stopped by subsequent addition of tet into the medium. By tagging the protein it will be degraded much faster by the Clp protease, so that the overall concentration is bounded and, after activating the stop-signal, the remaining proteins disappear quickly.<br> | The simulations show that our system actually should create a nice pulse-shaped expression of the fluorescent protein (GFP). This expression can be initiated by inducing with IPTG and stopped by subsequent addition of tet into the medium. By tagging the protein it will be degraded much faster by the Clp protease, so that the overall concentration is bounded and, after activating the stop-signal, the remaining proteins disappear quickly.<br> | ||
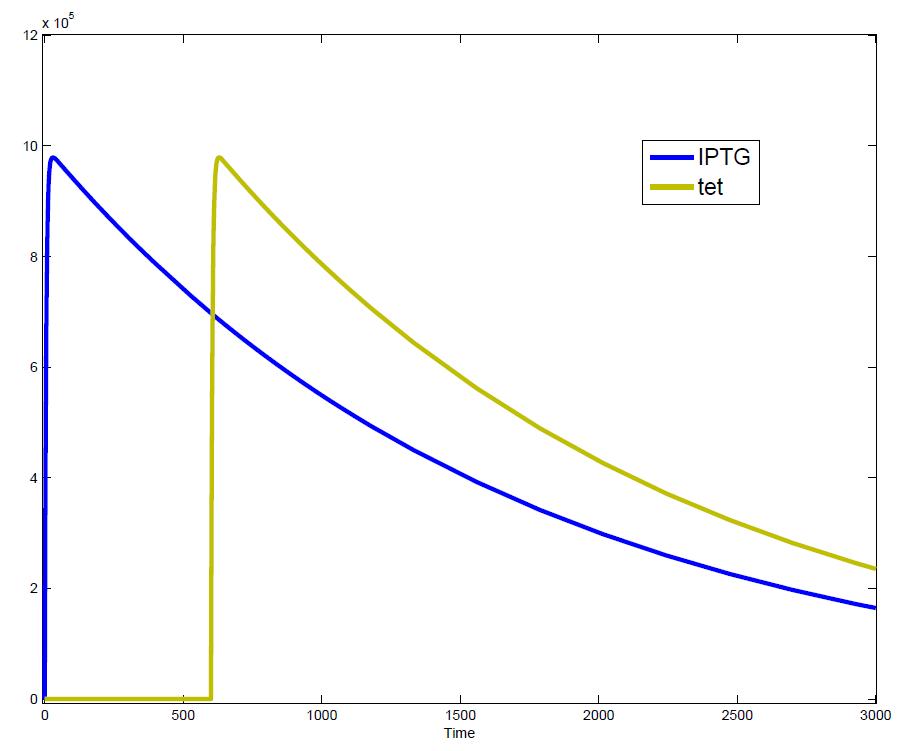
| - | Another fact that the simulations showed is that in order to get one single pulse the tet-concentration inside the medium must not reach zero before all the IPTG is degraded too. Otherwise there would still be IPTG in the system inhibiting the binding of LacI to the GFP-promoter and leading to an unwanted expression of our protein of interest as the LacIIs degrades.<br> | + | Another fact that the simulations showed is that in order to get one single pulse the tet-concentration inside, the medium must not reach zero before all the IPTG is degraded too. Otherwise there would still be IPTG in the system inhibiting the binding of LacI to the GFP-promoter and leading to an unwanted expression of our protein of interest as the LacIIs degrades.<br> |
| - | One way to overcome this problem is by simply inducing with a much higher quantity of tet than IPTG, so that it simply takes longer for it to completely degrade or being washed away. Still another way would be to wait a bit longer after the induction with IPTG so that it has already partly vanished until switching off the GFP-expression with tet. | + | One way to overcome this problem is by simply inducing with a much higher quantity of tet than IPTG, so that it simply takes longer for it to completely degrade or being washed away. Still another way would be to wait a bit longer after the induction with IPTG so that it has already partly vanished until switching off the GFP-expression by inducing with tet. |
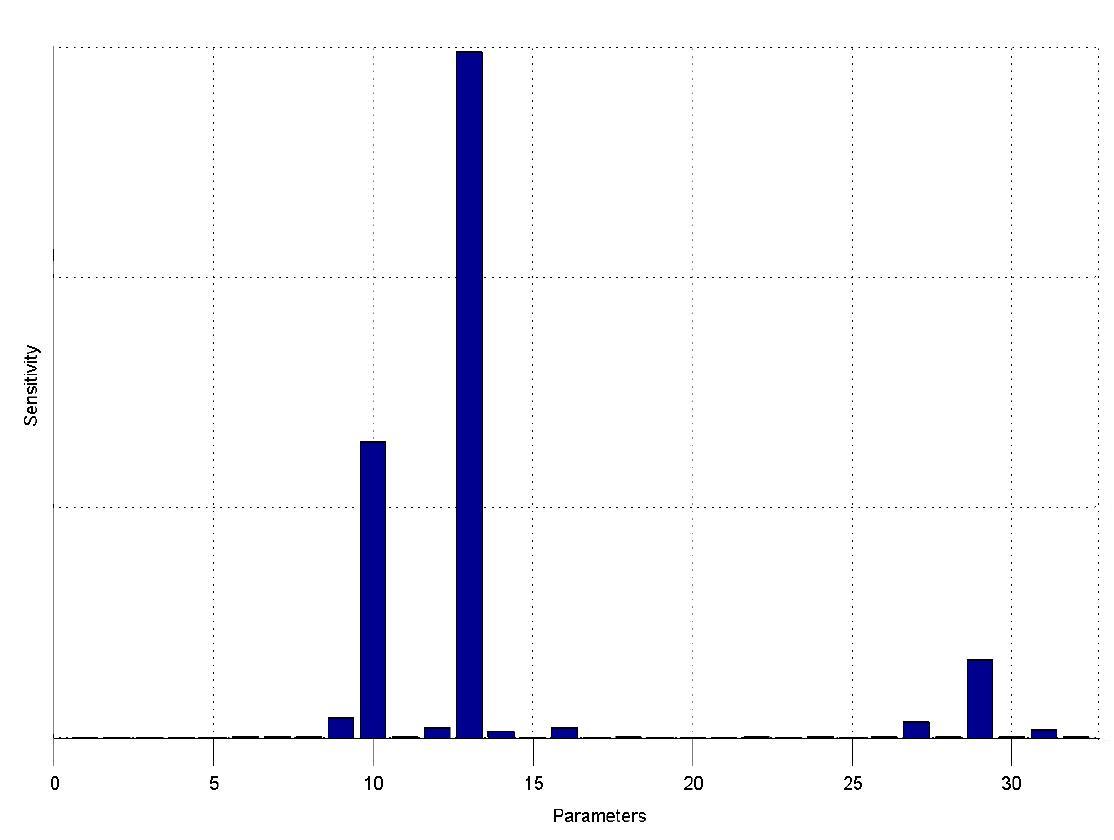
=== Sensitivity Analysis === | === Sensitivity Analysis === | ||
| Line 50: | Line 50: | ||
[[Team:ETH_Zurich/Modeling/Switch_Circuit#Parameters|parameters]]. | [[Team:ETH_Zurich/Modeling/Switch_Circuit#Parameters|parameters]]. | ||
| - | [[Image:sensitivities.JPG|thumb|center|600px|Sensitivity analysis - change in the GFP concentration depending on the change of the kinetic | + | [[Image:sensitivities.JPG|thumb|center|600px|Sensitivity analysis - change in the GFP concentration depending on the change of the kinetic parameters]] |
<br clear="all" > | <br clear="all" > | ||
The sensitivity analysis shows, that the concentration of the fluorescent protein strongly depends on its decay rate (parameter 13) the decay rate of its mRNA (parameter 10) and of course the transcription and translation rates of the protein (parameters 29 and 27), which is no surprise. | The sensitivity analysis shows, that the concentration of the fluorescent protein strongly depends on its decay rate (parameter 13) the decay rate of its mRNA (parameter 10) and of course the transcription and translation rates of the protein (parameters 29 and 27), which is no surprise. | ||
| - | We can also see that the decay | + | We can also see that the decay rates of LacIIs (parameter 9) and LacIIs_mRNA (parameter 12) and the transcription rate of LacIIs (parameter 31) have an influence on the expression of the fluorescent protein. |
<br clear="all" > | <br clear="all" > | ||
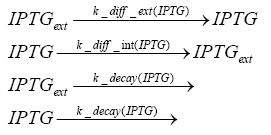
| Line 62: | Line 62: | ||
In order to switch on the circuit, we induce with IPTG. When IPTG is added into the medium it diffuses reversibly between the medium and the cells, where it is slowly degraded. In a chemostat extracellular IPTG is washed away.<br> | In order to switch on the circuit, we induce with IPTG. When IPTG is added into the medium it diffuses reversibly between the medium and the cells, where it is slowly degraded. In a chemostat extracellular IPTG is washed away.<br> | ||
| - | |||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
| Line 73: | Line 72: | ||
=== diffusion of tet === | === diffusion of tet === | ||
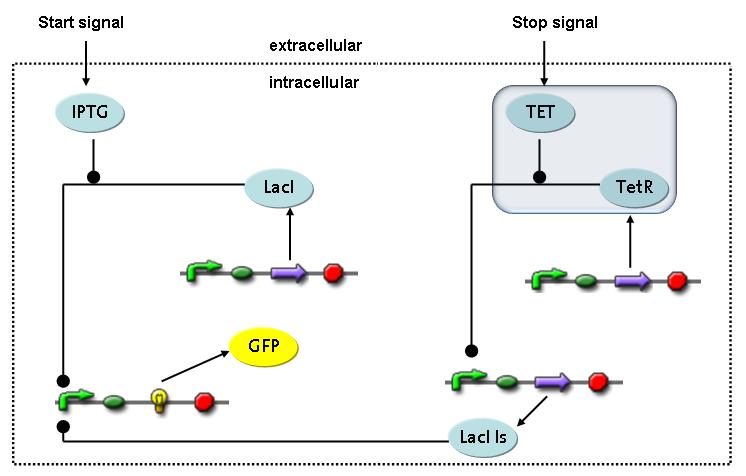
| - | The second inducer which is used in our system is tet. This one also diffuses | + | The second inducer which is used in our system is tet. This one also diffuses reversibly between the medium and the cells, where it is slowly degraded and is washed away in the medium. |
| - | + | ||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
| Line 84: | Line 82: | ||
<br clear="all" > | <br clear="all" > | ||
| - | === binding of | + | === binding of IPTG to LacI === |
| - | + | The first inducer IPTG can bind to the tetramerized LacI [https://2008.igem.org/Team:ETH_Zurich/Modeling/Switch_Circuit#References (4)].<br> | |
| - | + | ||
| - | + | ||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
<col width="*"> | <col width="*"> | ||
</colgroup> | </colgroup> | ||
| - | <tr width="950px"><td></html>[[Image: | + | <tr width="950px"><td></html>[[Image:Circuit_IPTG_LacI.JPG|thumb|250px]]<html></td><td></html> |
| - | [[Image: | + | [[Image:binding_IPTG_to_LacI.JPG|left]] |
<html></td></tr></table></html> | <html></td></tr></table></html> | ||
<br clear="all" > | <br clear="all" > | ||
| - | === binding of | + | === binding of tet to TetR === |
| - | + | The second inducer tet can bind to TetR.<br> | |
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
<col width="*"> | <col width="*"> | ||
</colgroup> | </colgroup> | ||
| - | <tr width="950px"><td></html>[[Image: | + | <tr width="950px"><td></html>[[Image:Circuit_tet_tetR.JPG|thumb|250px]]<html></td><td></html> |
| - | [[Image: | + | [[Image:binding_tet_to_tetR.JPG|left]] |
| - | + | ||
<html></td></tr></table></html> | <html></td></tr></table></html> | ||
<br clear="all" > | <br clear="all" > | ||
| - | === binding of | + | === binding of TetR to LacIIs-promoter === |
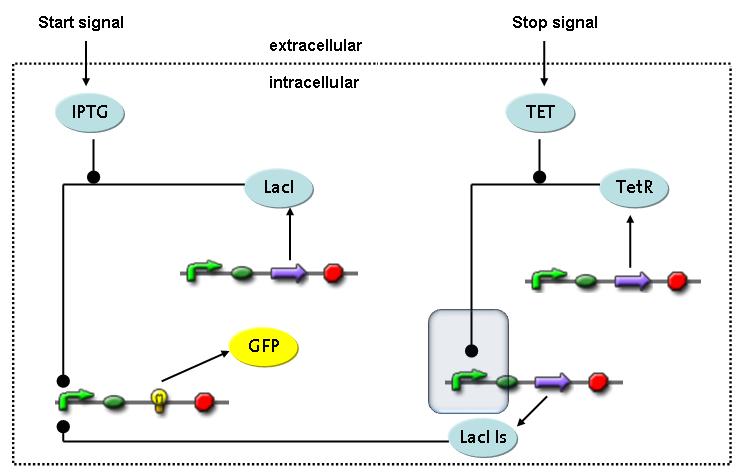
| - | + | TetR is constitutively expressed and binds to the LacIIs promoter, inhibiting its expression.<br> | |
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
<col width="*"> | <col width="*"> | ||
</colgroup> | </colgroup> | ||
| - | <tr width="950px"><td></html>[[Image: | + | <tr width="950px"><td></html>[[Image:Circuit_TetR_PLacIIs.JPG|thumb|250px]]<html></td><td></html> |
| - | [[Image: | + | [[Image:binding_tetR_to_LacIIs.JPG|left]] |
<html></td></tr></table></html> | <html></td></tr></table></html> | ||
<br clear="all" > | <br clear="all" > | ||
| - | === binding of | + | === binding of LacI and LacIIs to GFP-promoter === |
| - | + | LacI which is constitutively expressed and LacIIs which is under the control of a tet repressor can bind both to the GFP promotor.<br> | |
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
<col width="*"> | <col width="*"> | ||
</colgroup> | </colgroup> | ||
| - | <tr width="950px"><td></html>[[Image: | + | <tr width="950px"><td></html>[[Image:Circuit_LacI_LacIIs_Pgfp.JPG|thumb|250px]]<html></td><td></html> |
| - | [[Image: | + | [[Image:binding_LacIIs_to_Pgfp.JPG|left]] |
| + | [[Image:binding_LacI_to_Pgfp.JPG|left]] | ||
<html></td></tr></table></html> | <html></td></tr></table></html> | ||
<br clear="all" > | <br clear="all" > | ||
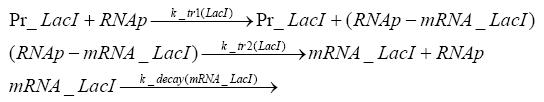
=== transcription and translation of LacI === | === transcription and translation of LacI === | ||
| - | + | * RNA polymerase binds to the LacI-promoter and transcribes it into LacI-mRNA | |
| + | * RNA polymerase detaches from the LacI-mRNA | ||
| + | * degradation of LacI-mRNA | ||
| + | * ribosome binds to LacI-mRNA and translates it into LacI | ||
| + | * ribosome detaches from LacI | ||
| + | * degradation of LacI | ||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
| Line 144: | Line 145: | ||
=== transcription and translation of LacIIs === | === transcription and translation of LacIIs === | ||
| - | + | * RNA polymerase binds to the LacIIs-promoter and transcribes it into LacIIs-mRNA | |
| + | * RNA polymerase detaches from the LacIIs-mRNA | ||
| + | * degradation of LacIIs-mRNA | ||
| + | * ribosome binds to LacIIs-mRNA and translates it into LacIIs | ||
| + | * ribosome detaches from LacIIs | ||
| + | * degradation of LacIIs | ||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
| Line 156: | Line 162: | ||
=== transcription and translation of TetR === | === transcription and translation of TetR === | ||
| - | + | * RNA polymerase binds to the TetR-promoter and transcribes it into TetR-mRNA | |
| + | * RNA polymerase detaches from the TetR-mRNA | ||
| + | * degradation of TetR-mRNA | ||
| + | * ribosome binds to TetR-mRNA and translates it into TetR | ||
| + | * ribosome detaches from TetR | ||
| + | * degradation of TetR | ||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
| Line 168: | Line 179: | ||
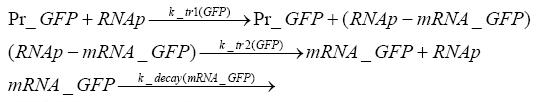
=== transcription and translation of GFP === | === transcription and translation of GFP === | ||
| - | + | * RNA polymerase binds to the GFP-promoter and transcribes it into GFP-mRNA | |
| + | * RNA polymerase detaches from the GFP-mRNA | ||
| + | * degradation of GFP-mRNA | ||
| + | * ribosome binds to TGFP-mRNA and translates it into GFP | ||
| + | * ribosome detaches from GFP | ||
| + | * degradation of GFP | ||
<html><table border=0> <colgroup> | <html><table border=0> <colgroup> | ||
<col width="250"> | <col width="250"> | ||
| Line 208: | Line 224: | ||
In this section you can find all the parameters used in the simulation.<br> | In this section you can find all the parameters used in the simulation.<br> | ||
| - | Because many of the in vivo rates of the biochemical reactions we simulated are unknown | + | Because many of the in vivo rates of the biochemical reactions we simulated are unknown or could not be found in the literature, the kinetic parameters were mainly obtained from estimates [https://2008.igem.org/Team:ETH_Zurich/Modeling/Switch_Circuit#References (2)] based on the values found in the supporting text to [https://2008.igem.org/Team:ETH_Zurich/Modeling/Switch_Circuit#References (1)].<br> |
{| border="1" | {| border="1" | ||
| Line 220: | Line 236: | ||
|- | |- | ||
! 3 | ! 3 | ||
| - | | k_assoc( | + | | k_assoc(LacIIs) || 5.0 || 1/(mole*second) || Estimate |
|- | |- | ||
! 4 | ! 4 | ||
| Line 238: | Line 254: | ||
|- | |- | ||
! 9 | ! 9 | ||
| - | | k_dec( | + | | k_dec(LacIIs) || 0.05 || 1/second || Estimate |
|- | |- | ||
! 10 | ! 10 | ||
| Line 247: | Line 263: | ||
|- | |- | ||
! 12 | ! 12 | ||
| - | | k_dec( | + | | k_dec(mRNA_LacIIs) || 0.05 || 1/second || Estimate |
|- | |- | ||
! 13 | ! 13 | ||
| Line 277: | Line 293: | ||
|- | |- | ||
! 22 | ! 22 | ||
| - | | k_dissoc( | + | | k_dissoc(LacIIs) || 1.0 || 1/second || Estimate |
|- | |- | ||
! 23 | ! 23 | ||
| Line 289: | Line 305: | ||
|- | |- | ||
! 26 | ! 26 | ||
| - | | k_tl( | + | | k_tl(LacIIs) || 5.0 || 1/second || Estimate |
|- | |- | ||
! 27 | ! 27 | ||
| Line 304: | Line 320: | ||
|- | |- | ||
! 31 | ! 31 | ||
| - | | k_tr( | + | | k_tr(LacIIs) || 0.1 || 1/second || [***] |
|- | |- | ||
! 32 | ! 32 | ||
| Line 312: | Line 328: | ||
[*] the degradation constants of the two inducers are bigger outside the cell, the effect that they are washed away in the chemostat is taken into account in those parameters<br> | [*] the degradation constants of the two inducers are bigger outside the cell, the effect that they are washed away in the chemostat is taken into account in those parameters<br> | ||
[**] degradation rates of gfp are higher because of the tagging <br> | [**] degradation rates of gfp are higher because of the tagging <br> | ||
| - | [***] need to be that high to account for the autorepression of LacI/ in order to get a low steady state concentration of LacI of about 50 proteins (3) | + | [***] need to be that high to account for the autorepression of LacI / in order to get a low steady state concentration of LacI of about 50 proteins (3) |
== References == | == References == | ||
Latest revision as of 05:32, 30 October 2008
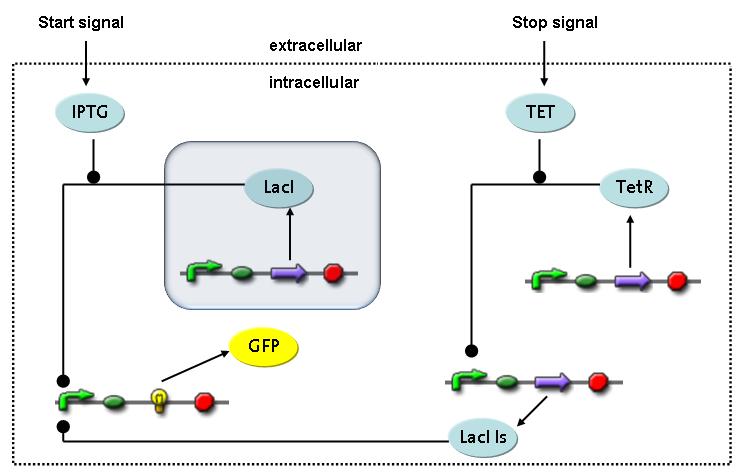
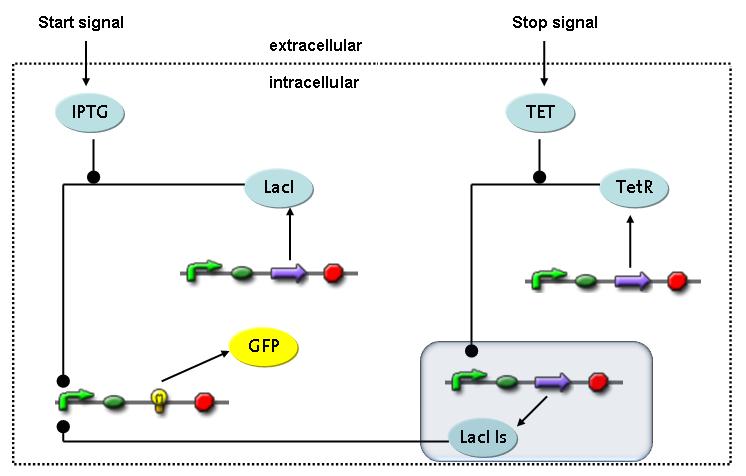
Switch CircuitThe designed switching circuit is driven by two input signals – a start signal initiates the synthesis of a specific protein and a terminating signal switches gene expression off. The goal of this system is to control expression of restriction enzymes in order to delete genome fragments in vivo.
In order to do some preliminary experiments, the restriction enzyme has been substituted by a fluorescent protein. The detailed mechanism is described here. It follows a summarized version of it's mode of operation: This switching circuit is described by a set of 64 chemical reactions and 41 molecular species. In order to do a computational analysis of the circuit, this model has been simplified and implemented in MATLAB. Then the system has been simulated using ODE and stochastic solvers. Implementation and SimulationImplementationIn the implementation, some minor parts that do not have significant effects on the aspired results have been neglected for the sake of simplicity. In the MATLAB-model of the different protein-expressions, the RNA-polymerase and the ribosomes have not been taken into account, in the transcription and translation respectively. Furthermore, the effects of the dimerization of TetR as well as the impacts of dimerization and tetramerization of LacI and LacIIs (4) have not been considered in the final implementation. This simplified model still comprises more than 20 different molecular species and over 30 kinetic reactions and was implemented by using the SimBiology Toolbox in MATLAB. We performed deterministic and stochastic simulations based on Mass Action Kinetics. The stochastic simulations turned out to be computationally very exhaustive but generated no further significant information compared to the deterministic simulations. Simulation Results
The simulations show that our system actually should create a nice pulse-shaped expression of the fluorescent protein (GFP). This expression can be initiated by inducing with IPTG and stopped by subsequent addition of tet into the medium. By tagging the protein it will be degraded much faster by the Clp protease, so that the overall concentration is bounded and, after activating the stop-signal, the remaining proteins disappear quickly. Another fact that the simulations showed is that in order to get one single pulse the tet-concentration inside, the medium must not reach zero before all the IPTG is degraded too. Otherwise there would still be IPTG in the system inhibiting the binding of LacI to the GFP-promoter and leading to an unwanted expression of our protein of interest as the LacIIs degrades. One way to overcome this problem is by simply inducing with a much higher quantity of tet than IPTG, so that it simply takes longer for it to completely degrade or being washed away. Still another way would be to wait a bit longer after the induction with IPTG so that it has already partly vanished until switching off the GFP-expression by inducing with tet. Sensitivity AnalysisWe define the sensitivity as the change of the production of the desired fluorescence protein - which is the output of our system - depending on the change of the parameters.
The sensitivity analysis shows, that the concentration of the fluorescent protein strongly depends on its decay rate (parameter 13) the decay rate of its mRNA (parameter 10) and of course the transcription and translation rates of the protein (parameters 29 and 27), which is no surprise.
We can also see that the decay rates of LacIIs (parameter 9) and LacIIs_mRNA (parameter 12) and the transcription rate of LacIIs (parameter 31) have an influence on the expression of the fluorescent protein.
Detailed Modeldiffusion of IPTGIn order to switch on the circuit, we induce with IPTG. When IPTG is added into the medium it diffuses reversibly between the medium and the cells, where it is slowly degraded. In a chemostat extracellular IPTG is washed away. diffusion of tetThe second inducer which is used in our system is tet. This one also diffuses reversibly between the medium and the cells, where it is slowly degraded and is washed away in the medium. binding of IPTG to LacIThe first inducer IPTG can bind to the tetramerized LacI (4). binding of tet to TetRThe second inducer tet can bind to TetR. binding of TetR to LacIIs-promoterTetR is constitutively expressed and binds to the LacIIs promoter, inhibiting its expression. binding of LacI and LacIIs to GFP-promoterLacI which is constitutively expressed and LacIIs which is under the control of a tet repressor can bind both to the GFP promotor. transcription and translation of LacI
transcription and translation of LacIIs
transcription and translation of TetR
transcription and translation of GFP
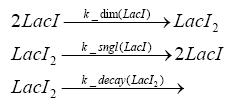
dimerization and tetramerization of LacI and LacIIsdimerization of tetRParametersIn this section you can find all the parameters used in the simulation. Because many of the in vivo rates of the biochemical reactions we simulated are unknown or could not be found in the literature, the kinetic parameters were mainly obtained from estimates (2) based on the values found in the supporting text to (1).
[*] the degradation constants of the two inducers are bigger outside the cell, the effect that they are washed away in the chemostat is taken into account in those parameters References(1) "Spatiotemporal control of gene expression with pulse-generating networks", Basu et al., PNAS, 2004 (2) "Genetic circuit building blocks for cellular computation, communications, and signal processing", Weiss et al., Natural Computing, 2003 (3) "Predicting stochastic gene expression dynamics in single cells", Mettetal et al., PNAS, 2006 (4) "Engineered gene circuits", Hasty et al., Nature, 2002 |
 "
"