Team:Chiba/Demo experiments:Receivers
From 2008.igem.org
(Difference between revisions)
(→results) |
(→results) |
||
| (7 intermediate revisions not shown) | |||
| Line 49: | Line 49: | ||
===Varying bacterial numbers-results and discussion=== | ===Varying bacterial numbers-results and discussion=== | ||
| - | =results= | + | ===results=== |
No Dilution | No Dilution | ||
| Line 78: | Line 78: | ||
0h 0.5h 1.0h 1.5h 2.0h | 0h 0.5h 1.0h 1.5h 2.0h | ||
| - | =discussion= | + | ===discussion=== |
| - | + | *We demonstrated that the GFP expression switch is delayed by the ratio of sender to receiver. | |
| - | * | + | *The result indicates that the amount of AHL from one bacterium per time is constant and independent of bacteria number density. |
| - | + | *This is probaly because the sender has no feedback circuit of AHL production. | |
| - | + | *Although this strategy can not change the time interval, we can manage the switch timing by changing the ratio of sender to receiver. | |
| - | + | ||
===Testing different receivers-results and discussion=== | ===Testing different receivers-results and discussion=== | ||
| - | =results= | + | ===results=== |
[[Image:Team-Chiba-IMG_0299.JPG.jpg|160px]] | [[Image:Team-Chiba-IMG_0299.JPG.jpg|160px]] | ||
| Line 96: | Line 95: | ||
1=N.C | 1=N.C | ||
| - | 2=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy) | + | 2=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:[https://2008.igem.org/Team:Chiba/Experiments:copy_number ptet-luxR-plux-GFP(high copy)] |
| - | 3=[https://2008.igem.org/Team:Chiba/Experiments:copy_number ptet-luxR-(low copy)],[http://partsregistry.org/Part:BBa_J37032 BBa_J37032 | + | 3=[https://2008.igem.org/Team:Chiba/Experiments:copy_number ptet-luxR-(low copy)],[http://partsregistry.org/Part:BBa_J37032 BBa_J37032 plux-GFP(high copy)] |
| - | 4=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy) | + | 4=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:[https://2008.igem.org/Team:Chiba/Experiments:copy_number ptet-luxR-plux-GFP(low copy)] |
5=[https://2008.igem.org/Team:Chiba/Experiments:LuxR_mutant ptet-mLuxR(too sensitive)-plux-GFP] | 5=[https://2008.igem.org/Team:Chiba/Experiments:LuxR_mutant ptet-mLuxR(too sensitive)-plux-GFP] | ||
| Line 108: | Line 107: | ||
7=[https://2008.igem.org/Team:Chiba/Project/Experiments:Signal_Molecule_Quencher ptet-luxR-plux-GFP-plac-aiiA] | 7=[https://2008.igem.org/Team:Chiba/Project/Experiments:Signal_Molecule_Quencher ptet-luxR-plux-GFP-plac-aiiA] | ||
| - | =discussion= | + | ===discussion=== |
| - | *2,3, | + | *Number 2,3 and 5 fluoresces in 30 min. We could not see time difference of these, it may be resulted from excess amount of sender bacteria. |
| - | + | *Precise experiments controlling the number of sender are necessary for further discussion. | |
| - | * | + | *In comparizon with number 2 (T9002:high copy), there was no change of fluorescence intensity of number 4 (T9002:low copy) in 4 hours. |
| - | + | *It is probably because of circuit working, since the AHL is provided enough for the receiver. | |
'''>[[Team:Chiba/Project#Demo Experiments|Back to the project page]]''' | '''>[[Team:Chiba/Project#Demo Experiments|Back to the project page]]''' | ||
{| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | {| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | ||
Latest revision as of 00:56, 30 October 2008
Contents |
Demo Experiment ~Receivers~
Varying bacterial numbers: method
- Receiver(T9002) pre-incubation
- Receiver:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002](JW1908)wascultured in 2mL LB-Amp (37°C,12h)
- Pre-incubated Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](JW1908))was plated so as to produce about 1000 colonies.
- Sender(S03623) pre-incubation
- Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908) was cultured in 50mL entrifuge tubes in 10mL of LB-Amp (37°C,12h)(2 tubes)
- Sender Wash
- Centrifuged 2 tubes containing([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](JW1908))at 20°C,3600rpm for 6min and discarded supernatant.
- Added 10mL LB-Amp to each tube.
- Repeated wash twice.
- Creating bacterial plates
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) tube 1 (10mL) was mixed with LB-Amp-agar(50°C)(10ml)to produce sender containing bacterialplate-1.
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) tube 2(100μl)was mixed with LB-Amp(9.9ml) and diluted 100-fold. 10ml of this solution was mixed with LB-Amp-agar(50°C)(10ml) and created Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908))containing bacterial plate-2.
- LB-Amp pre-cultured Sender solution-2(10μl) and LB-Amp(9.99ml)was mixed to dilute 1000-fold.10ml of this solution and LB-Amp-agar(50°C)(10ml) was mixed to create Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908) containing bacterial plate-3
- Lifted with nitrocellulose
- Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](JW1908))colony was transfered to a nitrocellulose filter and placed on each of Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908))containing bacterial plate (1~3) and Sender-absent negative control plate (t=0). Determined the time required for the colonies to fluoresce depending on the bacterial concentration (100 and 1000-fold dilution).
- Method to detect fluorescence
- Plates cultured at 37°C were exposed to UV (312nm) light once every 30 minutes to observe GFP fluorescence.
Testing different receivers-methods
- Receiver&sender pre-culture
- Used Receivers were:
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy)
- ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032]:plux-GFP(high copy)
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy)
- ptet-mLuxR(too sensitive)-plux-GFP
- ptet-luxR-plux-GFP-plac-aiiA
- (all JW1908)Each was cultured in 2ml LB (37°C,12h) and plated so that about 1000 colonies of receiver cells will grow.
- Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908) was cultured in 10mL LB in 50mL centrifuge tubes (37°C,12h)
- Used Receivers were:
- sender wash
- Each receiver-containing medium was centrifuged in 50mL tubes at de20°C, 3600rpm for 6min and supernatant discarded.
- Added 10mL LB to each tube.
- Repeated wash twice.
- Creating bacterial plates
- LB pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) tube 1 (10mL) was mixed with LB-agar(50°C)(10ml)to produce sender containing bacterial plate-1.
- LB pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) tube 2(100μl)was mixed with LB(9.9ml) and diluted 100-fold. 10ml of this solution was mixed with LB-agar(50°C)(10ml) and created Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) containing bacterial plate-2.
- LB pre-cultured Sender solution-2(10μl) and LB(9.99ml) was mixed to dilute 1000-fold.10ml of this solution and LB-agar(50°C)(10ml) was mixed to create Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) containing bacterial plate-3
- Lifted with nitrocellulose
- Each Receiver colony was transfered to a nitrocellulose filter and placed on a Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](JW1908)) containing bacterial plate (1~3) or a sender-absent negative control plate(t=0) to observe how receiver type affects the time taken for the colonies to display visible fluorescence.
- Method to detect fluorescence
- Plates cultured at 37°C were exposed to UV (312nm) light once every 30 minutes to observe GFP fluorescence.
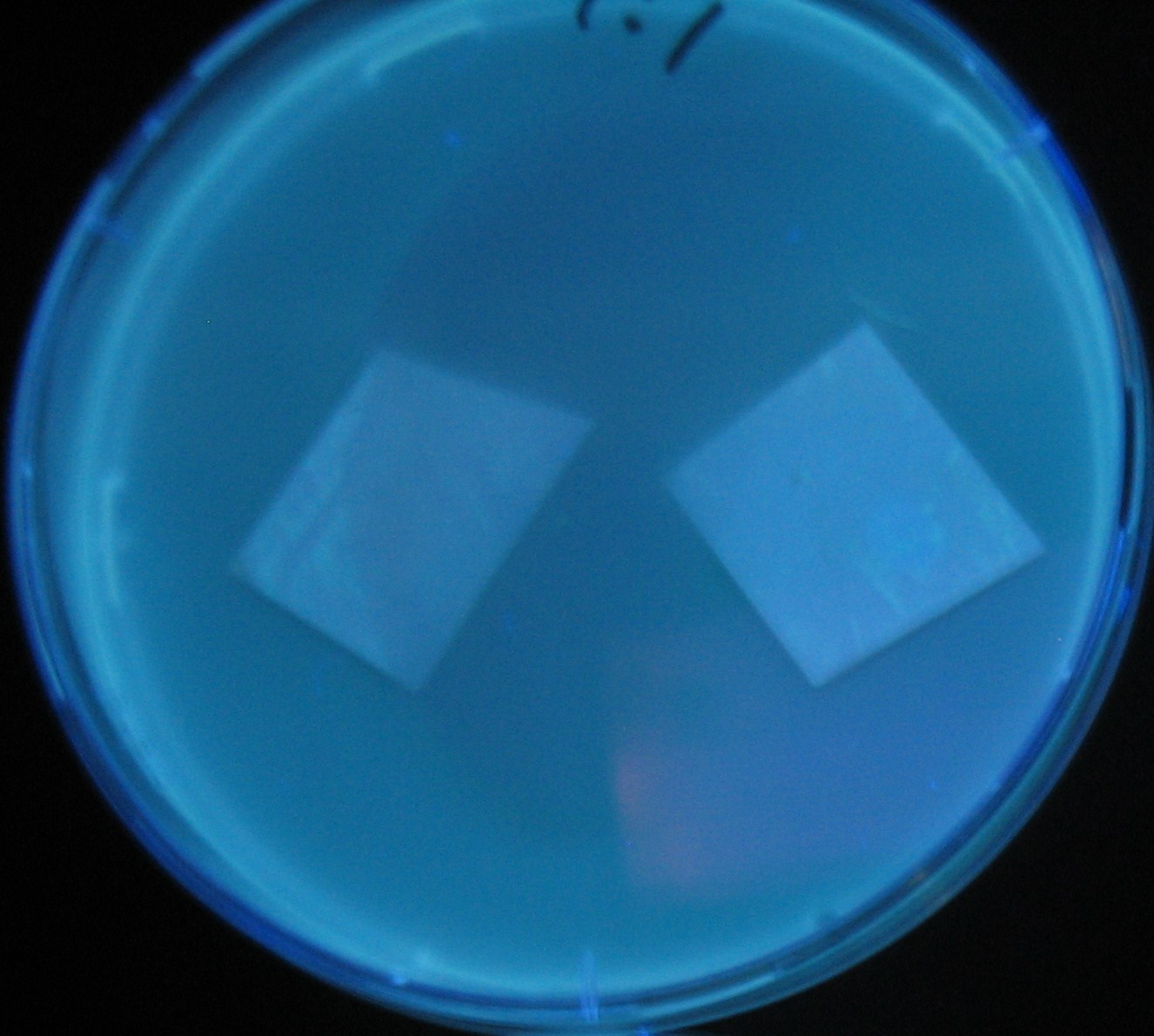
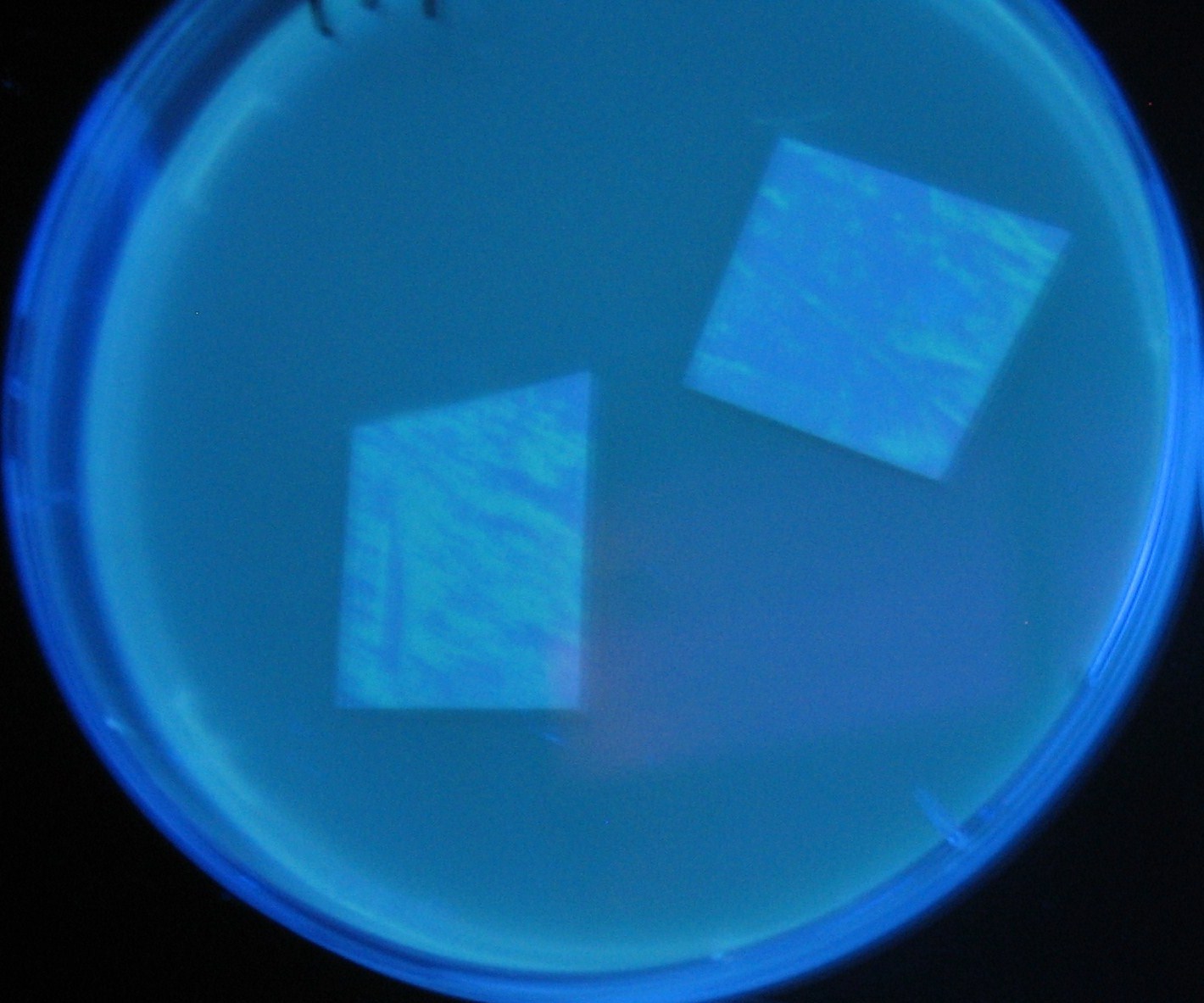
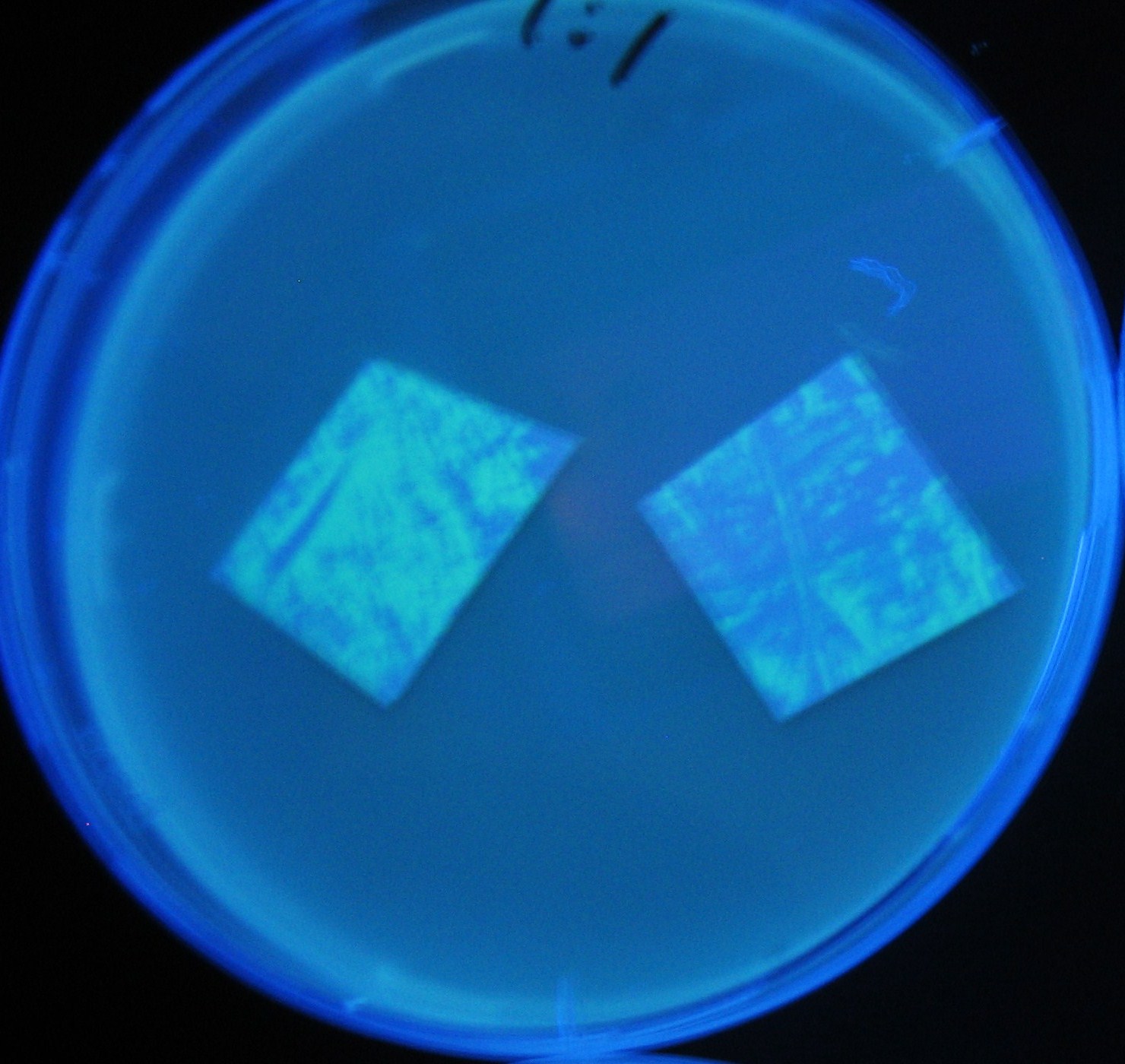
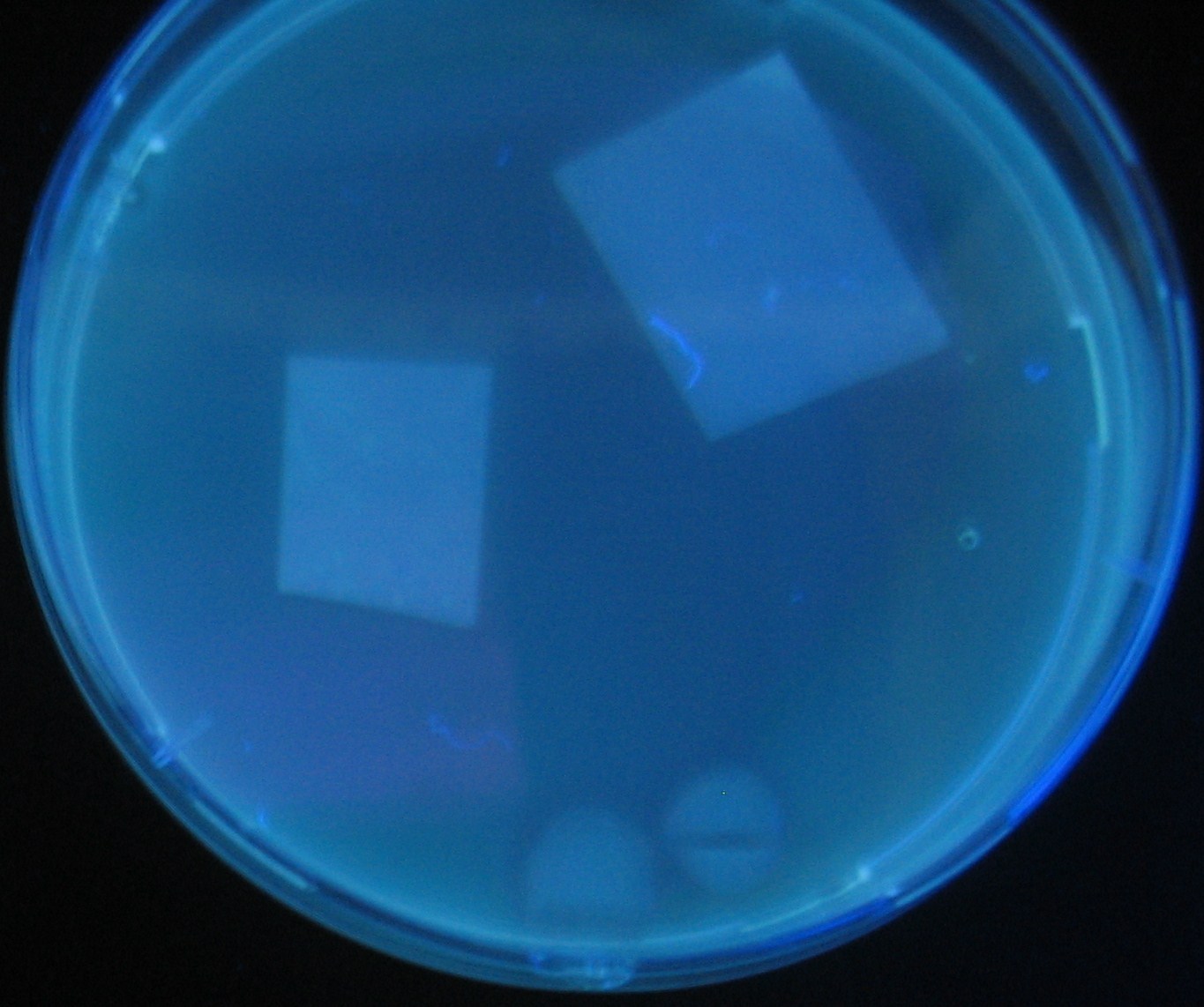
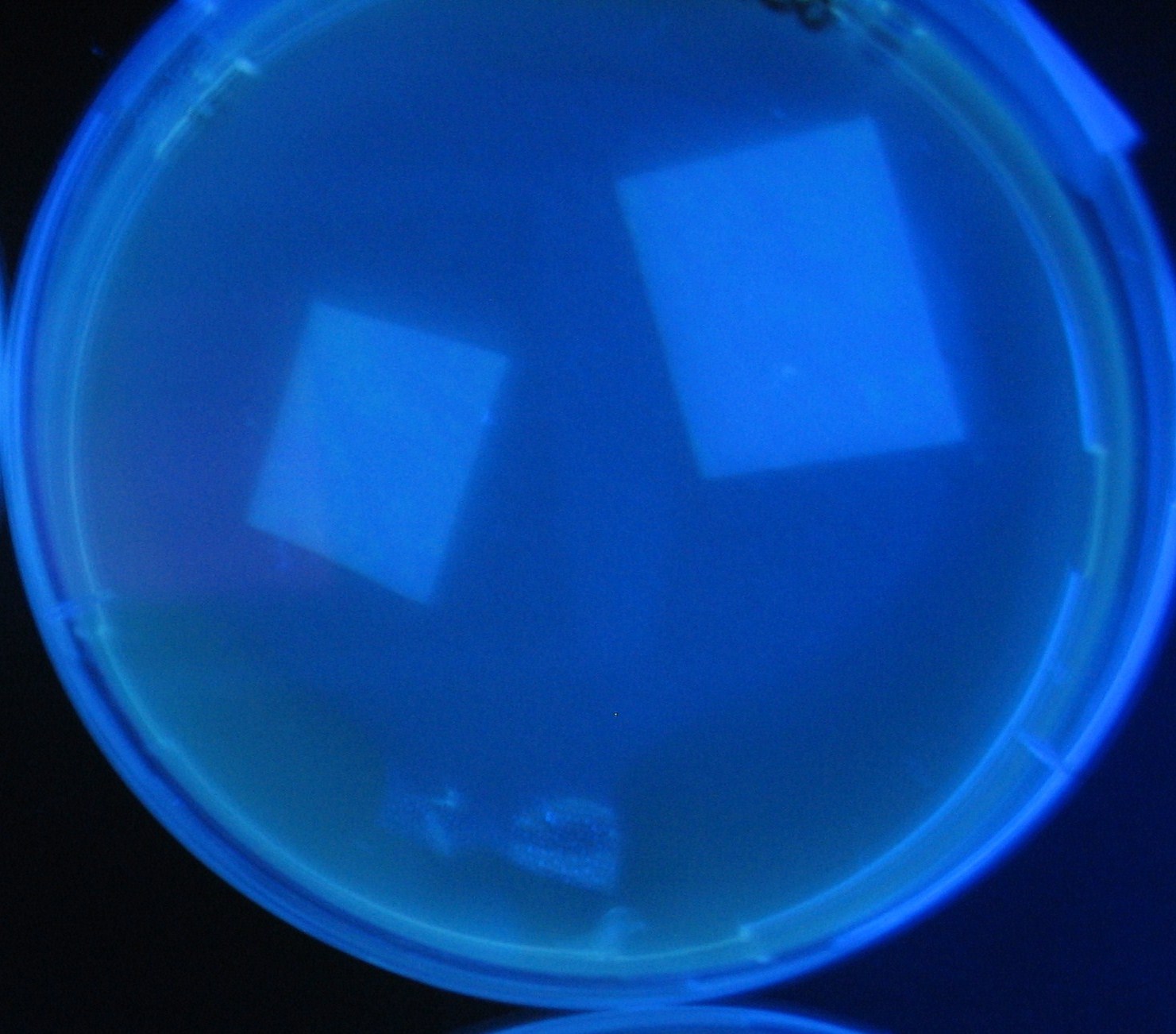
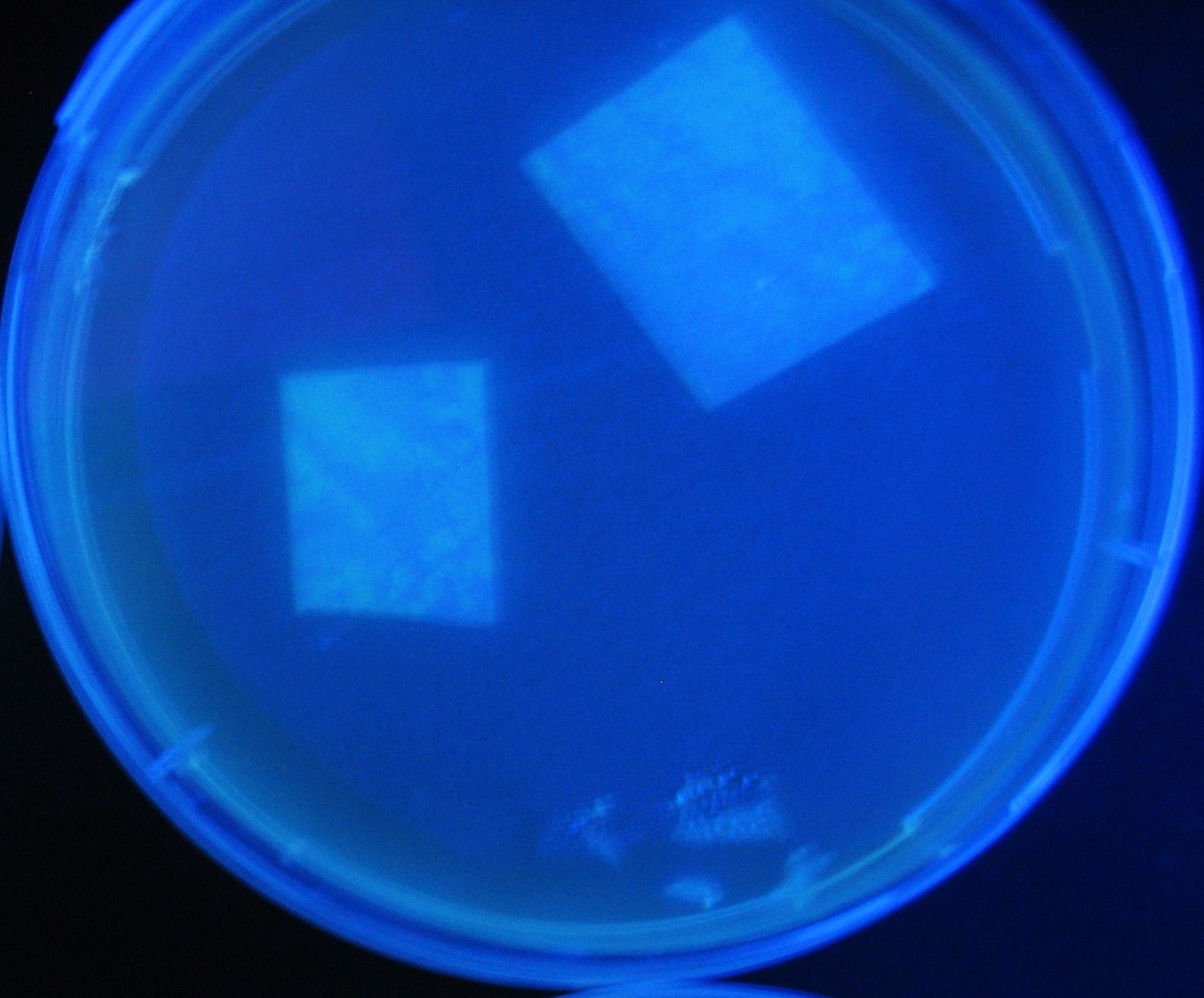
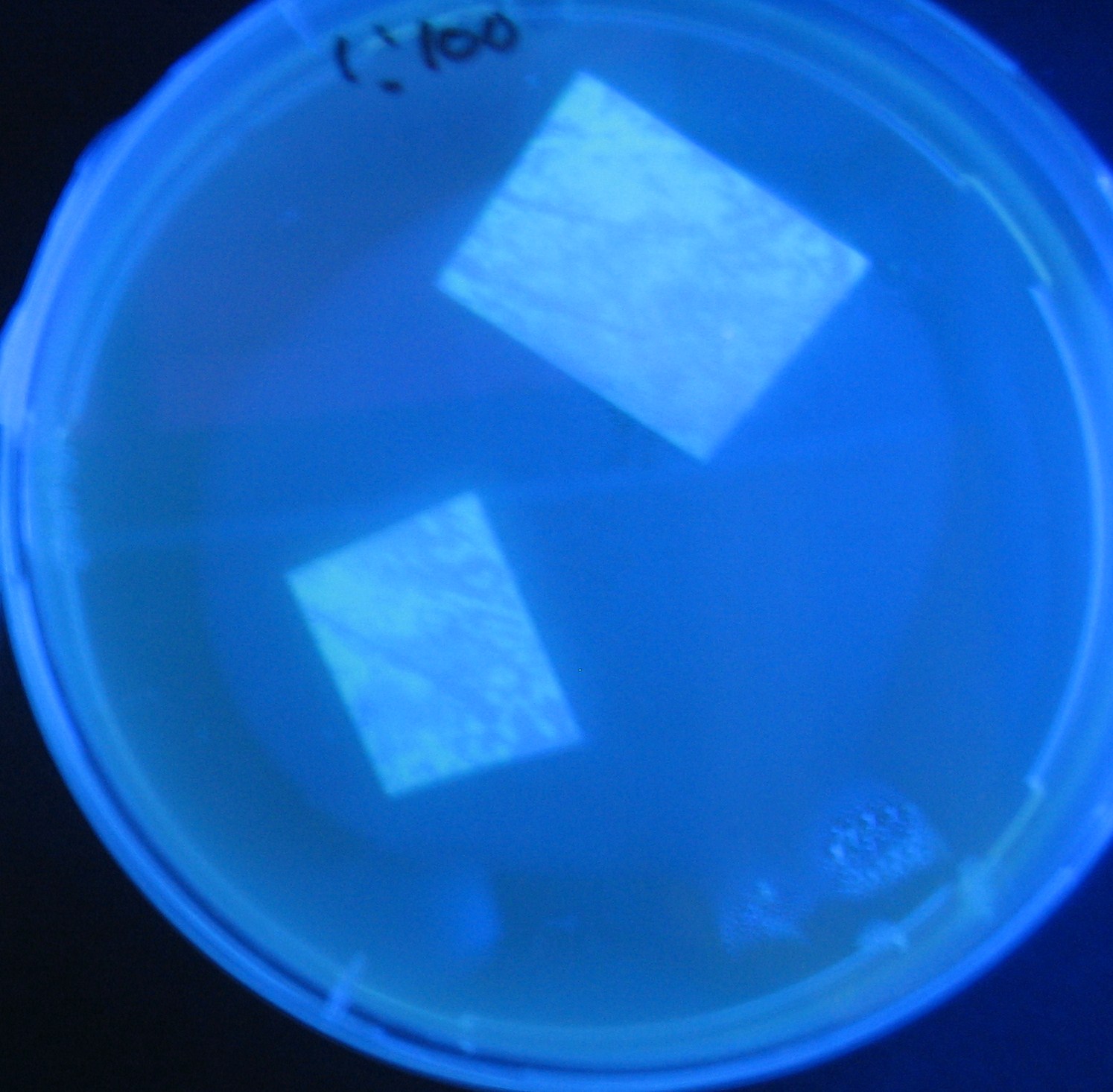
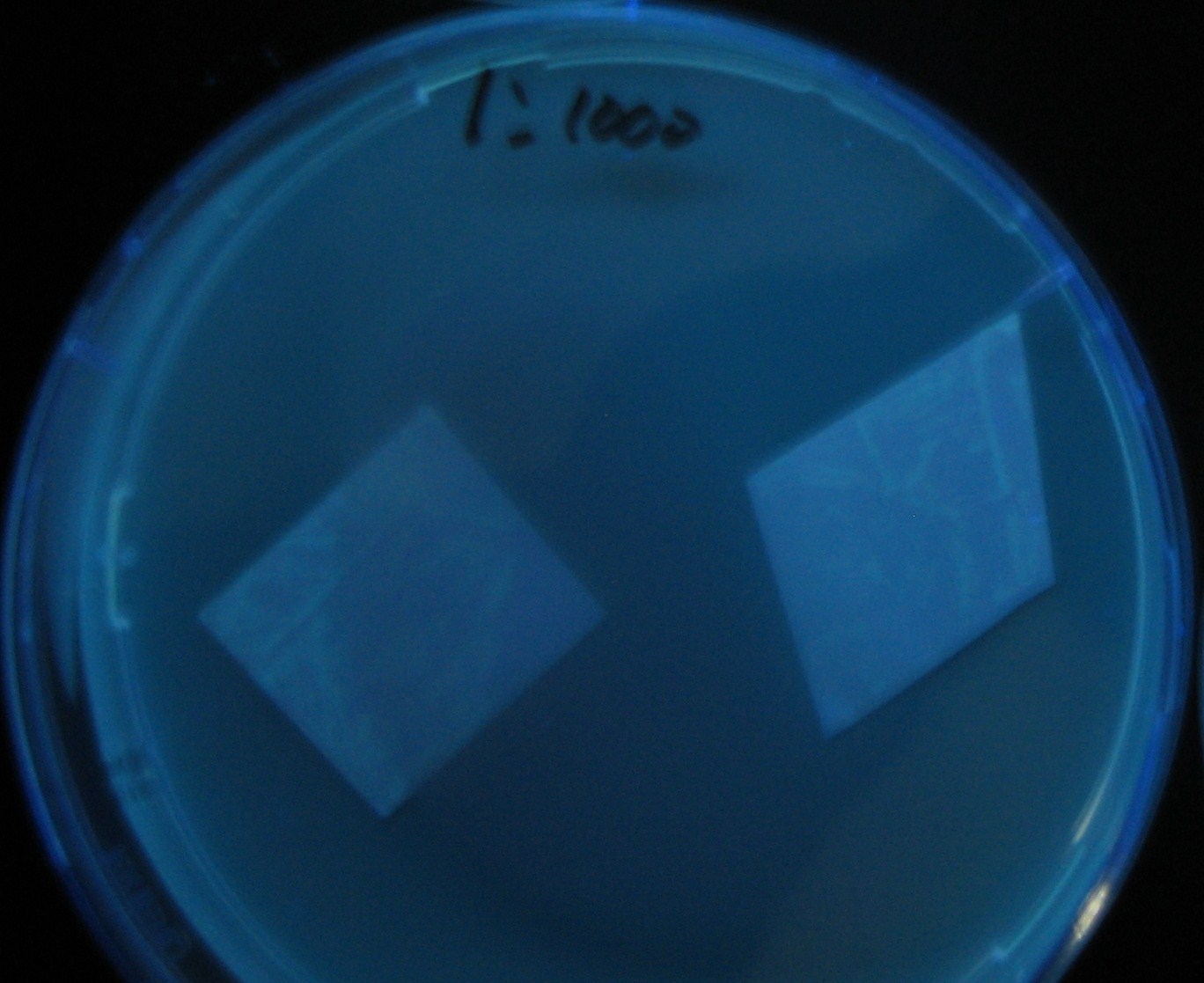
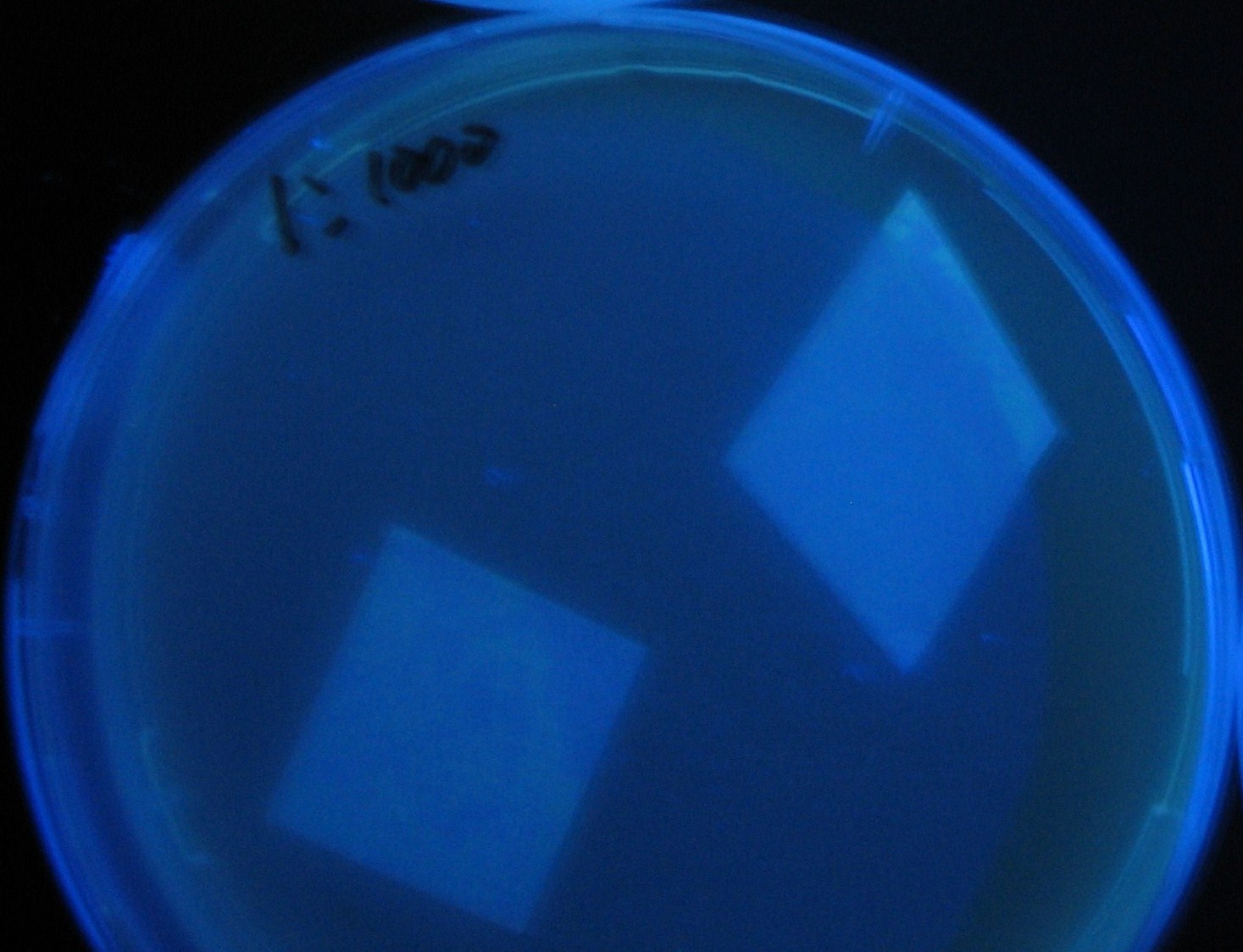
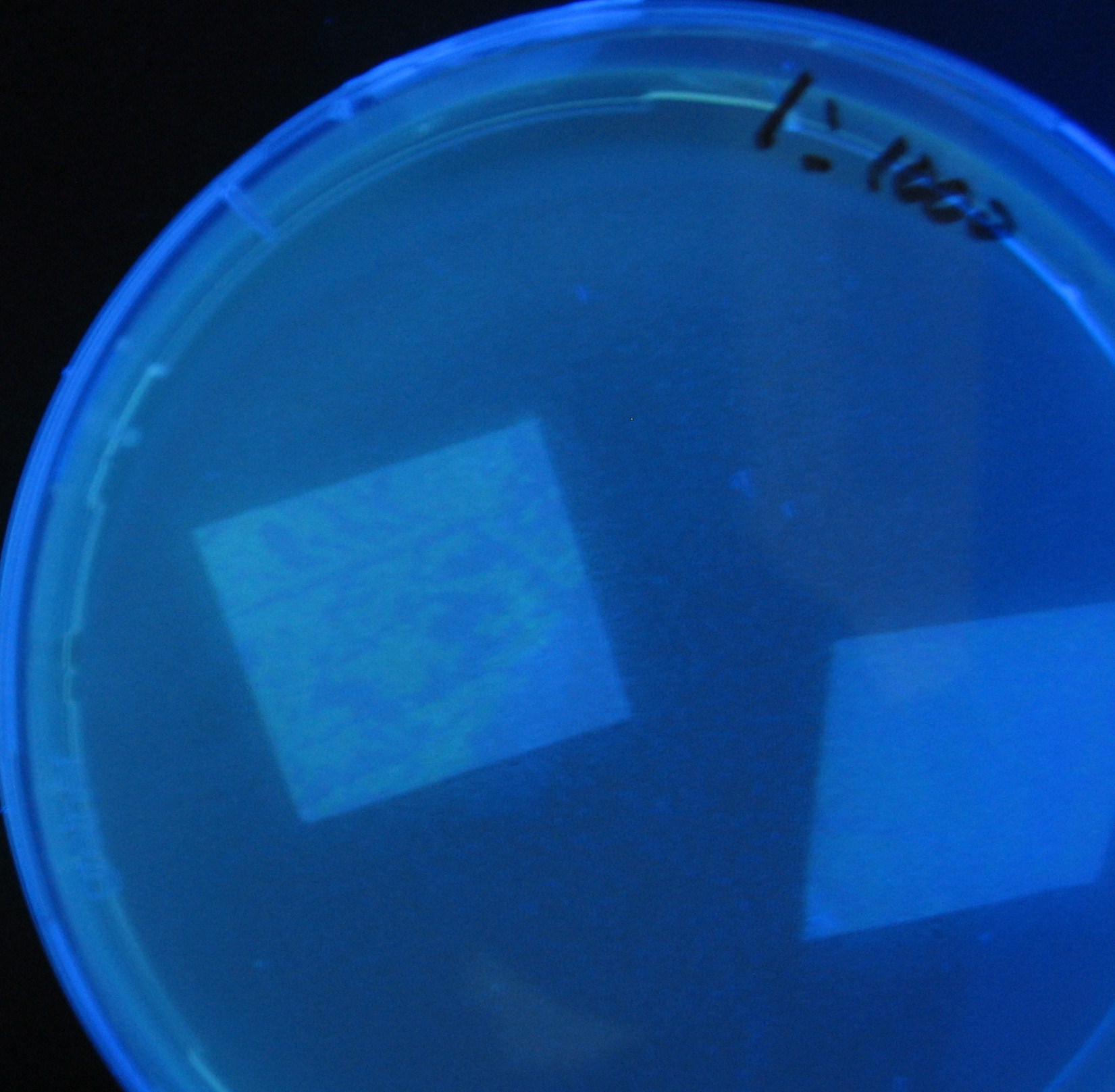
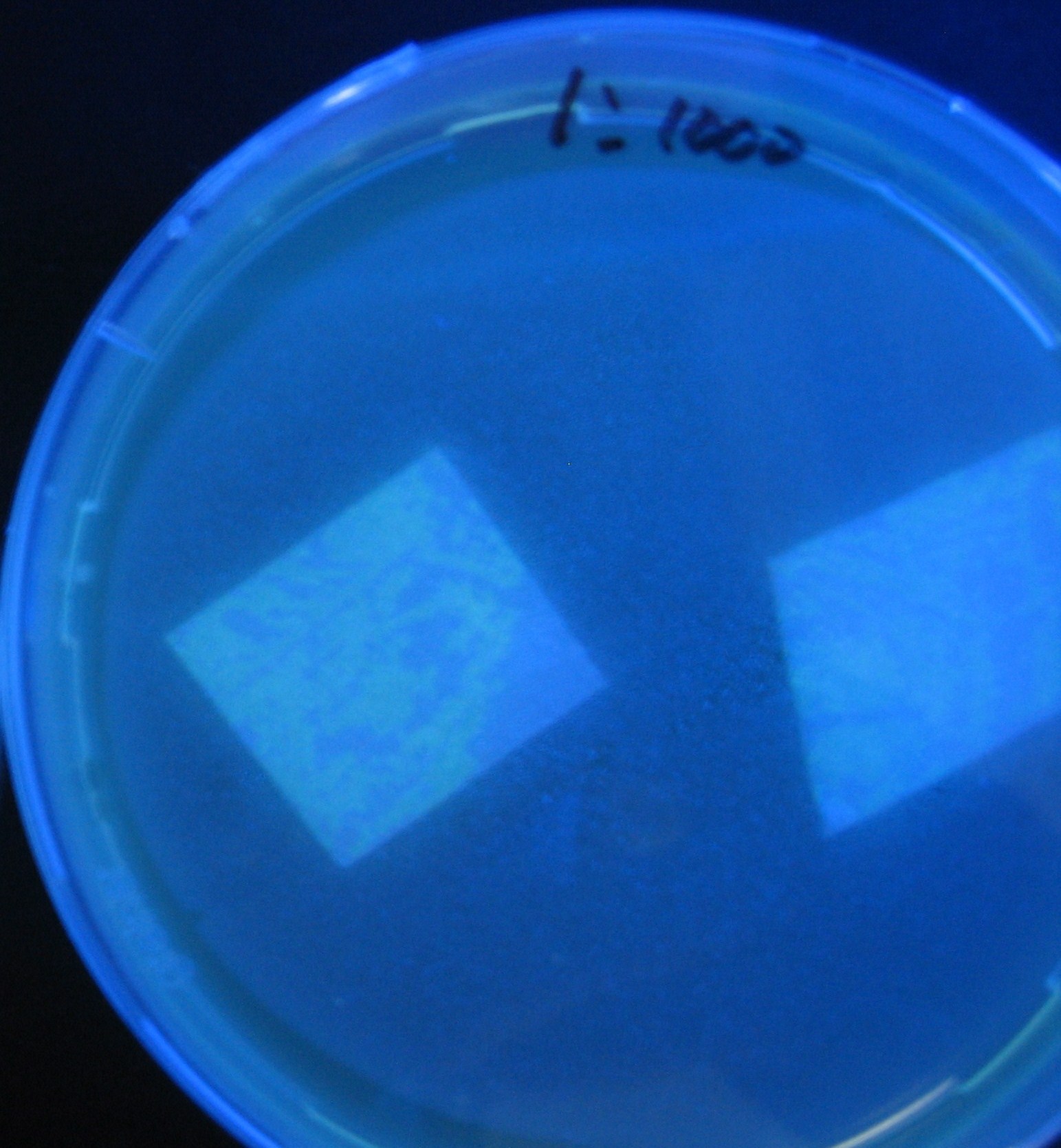
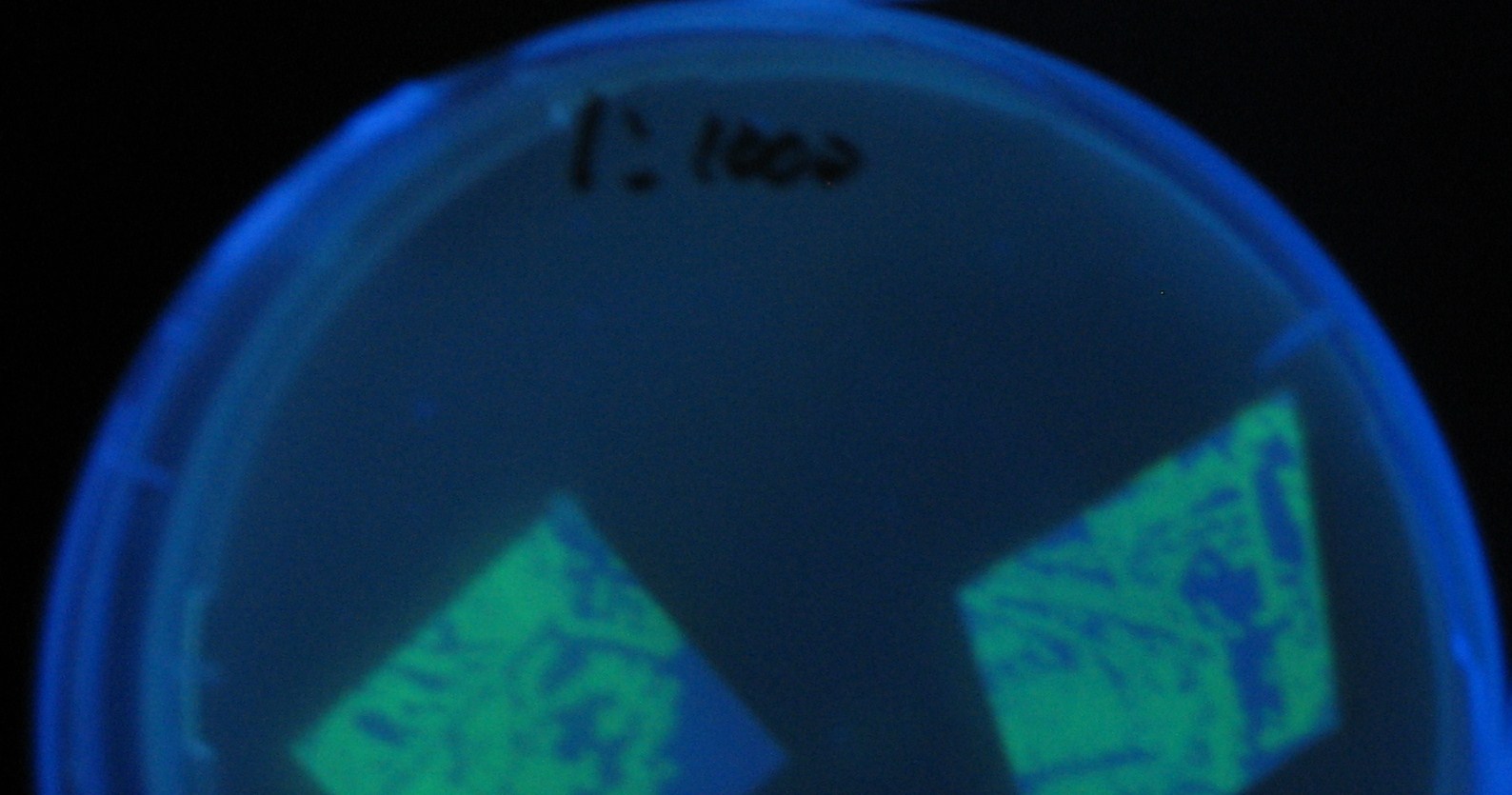
Varying bacterial numbers-results and discussion
results
0h 0.5h 1.0h
0h 0.5h 1.0h 1.5h
0h 0.5h 1.0h 1.5h 2.0h
discussion
- We demonstrated that the GFP expression switch is delayed by the ratio of sender to receiver.
- The result indicates that the amount of AHL from one bacterium per time is constant and independent of bacteria number density.
- This is probaly because the sender has no feedback circuit of AHL production.
- Although this strategy can not change the time interval, we can manage the switch timing by changing the ratio of sender to receiver.
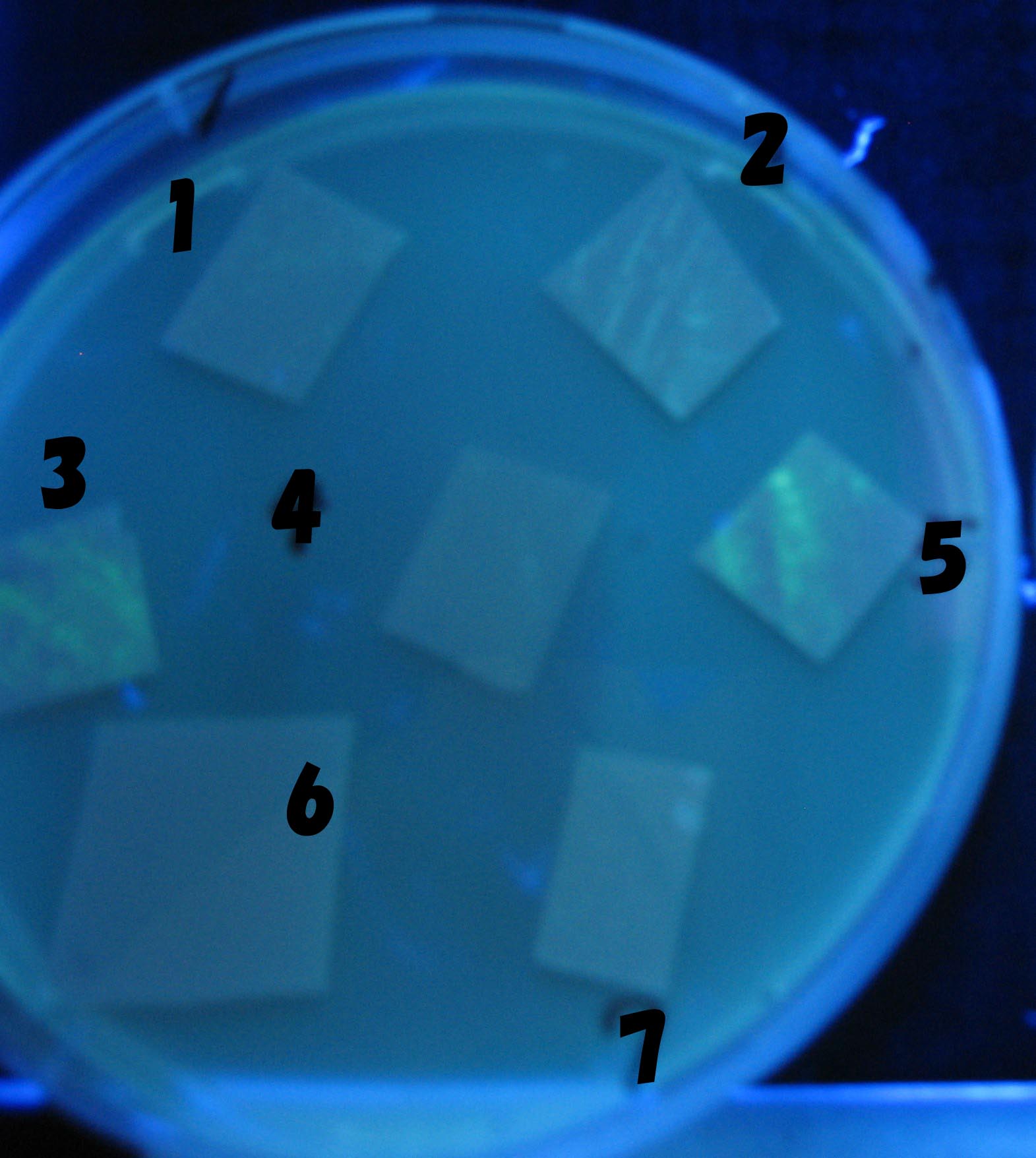
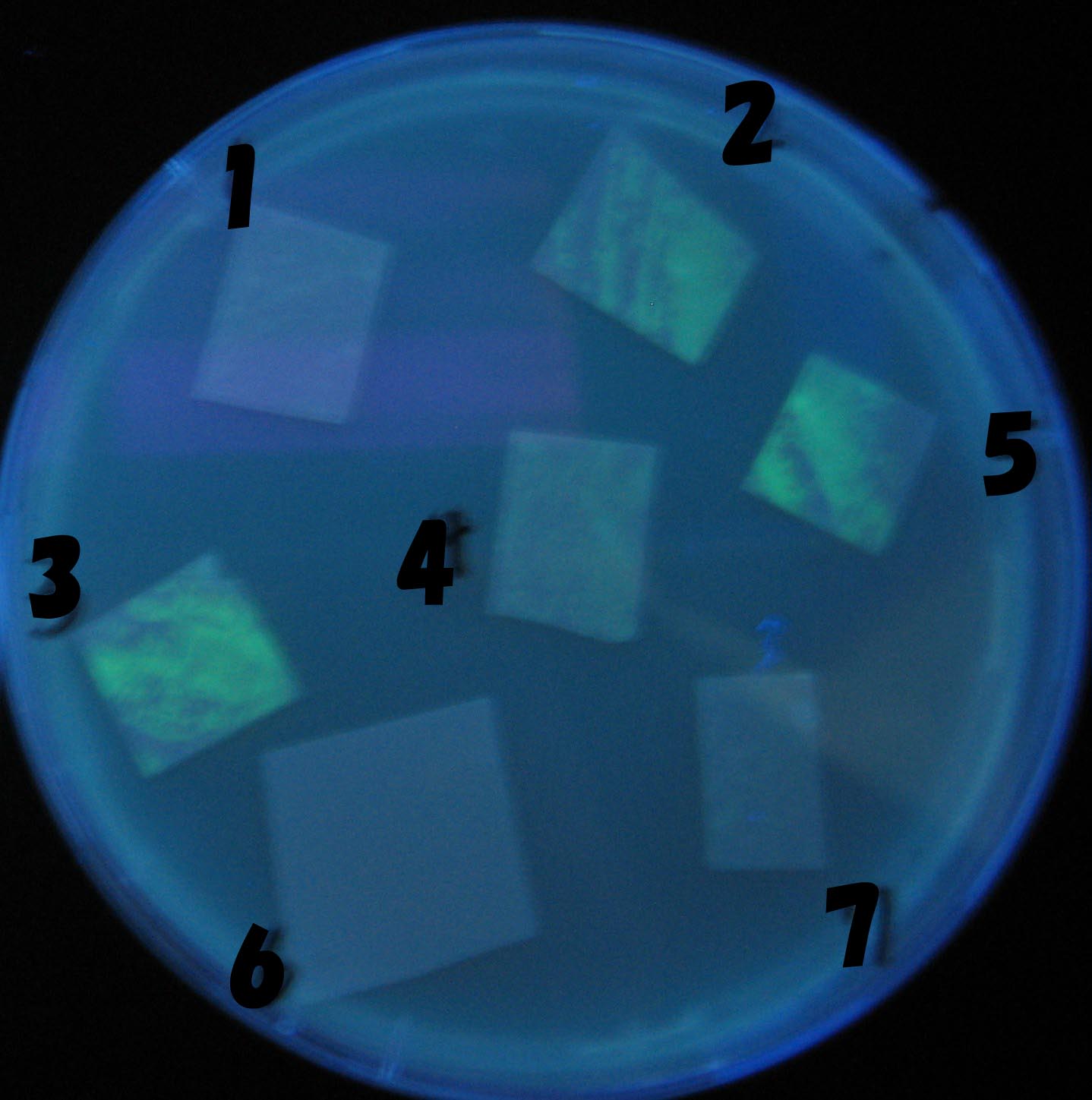
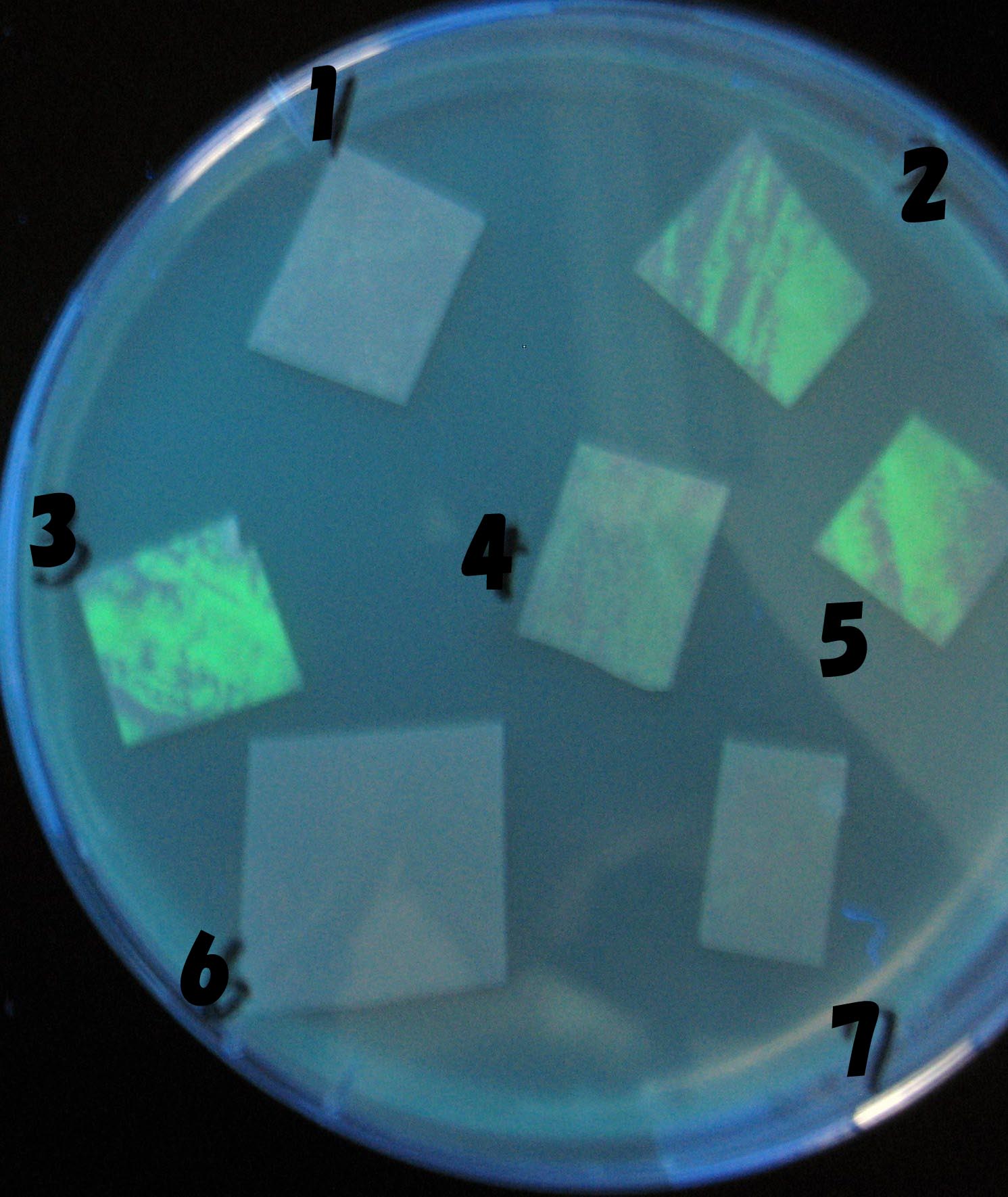
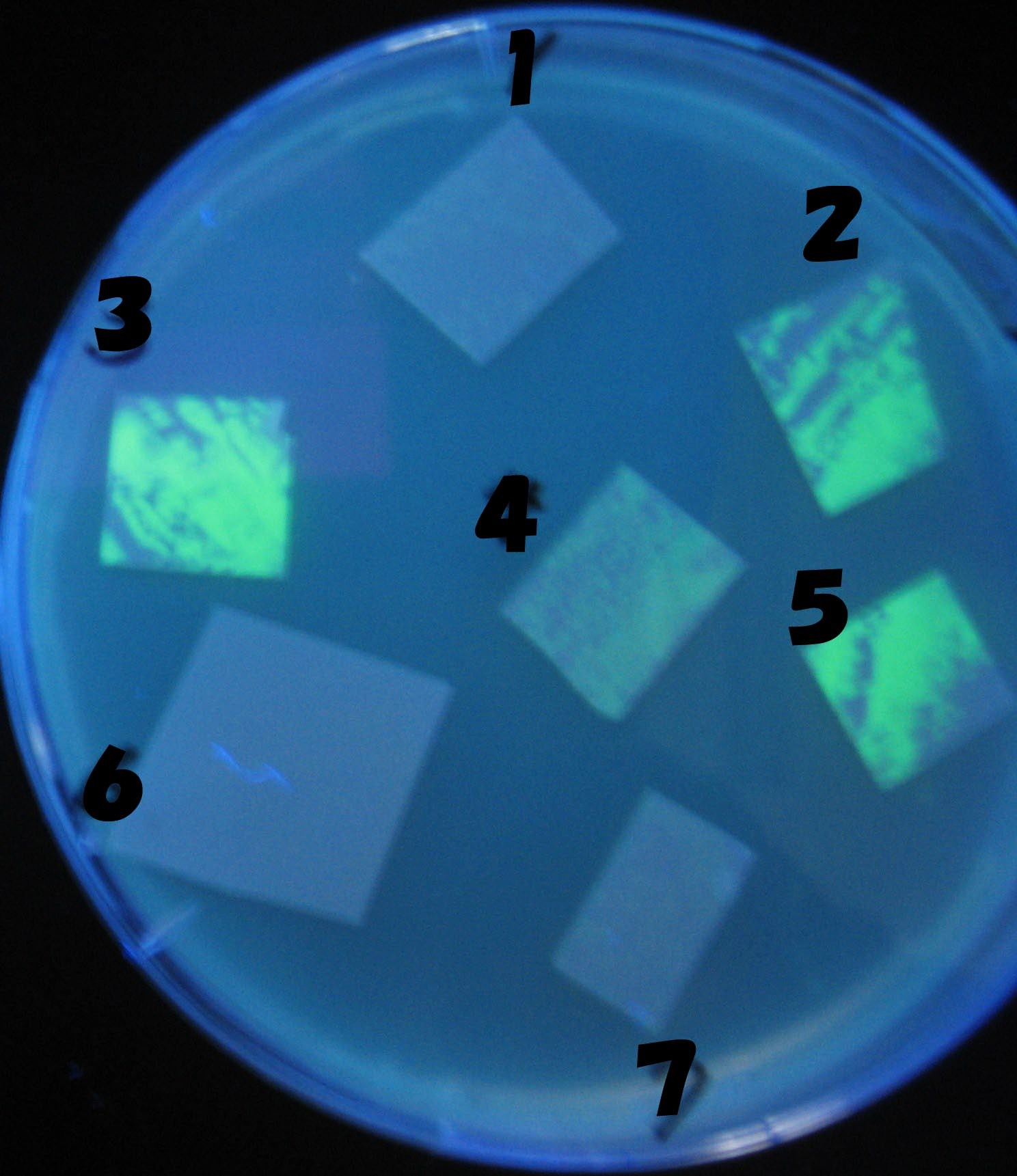
Testing different receivers-results and discussion
results
0h 0.5h 1.0h 1.5h
1=N.C
2=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy)
3=ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032 plux-GFP(high copy)]
4=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy)
5=ptet-mLuxR(too sensitive)-plux-GFP
6=N.C
7=ptet-luxR-plux-GFP-plac-aiiA
discussion
- Number 2,3 and 5 fluoresces in 30 min. We could not see time difference of these, it may be resulted from excess amount of sender bacteria.
- Precise experiments controlling the number of sender are necessary for further discussion.
- In comparizon with number 2 (T9002:high copy), there was no change of fluorescence intensity of number 4 (T9002:low copy) in 4 hours.
- It is probably because of circuit working, since the AHL is provided enough for the receiver.
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
 "
"