Team:Chiba/Experiments:LuxR mutant
From 2008.igem.org
(Difference between revisions)
(→Design) |
(→Experiment) |
||
| Line 35: | Line 35: | ||
'''Mutant construction:'''<br> | '''Mutant construction:'''<br> | ||
Using sewing PCR, we introduced each of three above mutations into LuxR. Some of the parts are await for sequence verification. | Using sewing PCR, we introduced each of three above mutations into LuxR. Some of the parts are await for sequence verification. | ||
| + | |||
'''Test communication: '''<br> | '''Test communication: '''<br> | ||
#Transformed sender (Ptet-luxI), mutant LuxR Receiver (Ptet-mLuxR-Plux-GFP) and WT LuxR Receiver into E. coli strains (JW1908) | #Transformed sender (Ptet-luxI), mutant LuxR Receiver (Ptet-mLuxR-Plux-GFP) and WT LuxR Receiver into E. coli strains (JW1908) | ||
Revision as of 03:08, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
LuxR mutant (Under Planning)
Design
Probably, the most straightforward approach to make variations in delay time is to create a number of LuxR mutants with different sensitivity. We thought the higher the sensitivity is, the shorter the delay time would be. The lower the sensitivity is, the slower the switch response should be.
At least, two papers on LuxR mutants (1),(2) are available. Among the many candidates, we chose the following three mutations;
- Ile45Phe: This mutation make LuxR 10x more sensitive to Lux-type AHL(1)
- Leu42Ala: Sensitivity (to AHL) becomes 1/15 of the wildtype LuxR(2)
- Leu42Ser: Sensitivity (to AHL) becomes 1/1,000 of the wildtype LuxR(2)
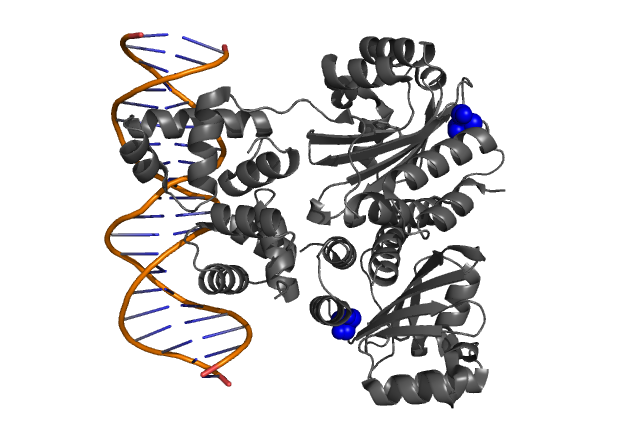
Fig. 3, Fig. 4 Hypothetical positions of residues in TraR corresponding to those found to modulate acyl-HSL specificity in LuxR. The crystal structure of the LuxR homologue TraR (PDB 1L3L) has been determined (Zhang et al., 2002).
Experiment
Mutant construction:
Using sewing PCR, we introduced each of three above mutations into LuxR. Some of the parts are await for sequence verification.
Test communication:
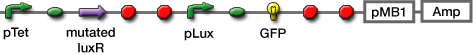
- Transformed sender (Ptet-luxI), mutant LuxR Receiver (Ptet-mLuxR-Plux-GFP) and WT LuxR Receiver into E. coli strains (JW1908)
- Separately inoculated Sender, WT Receiver (wild type luxR/BW⊿FliC) and mutated Receiver (1point mutation/BW⊿FliC) in liquid media for 12 h at 37℃.
- Inoculated again in liquid media upto about OD600=2 at 37℃
- Washed, adjusted the cell density, and mixed them. (Sender:Receiver=1000μL:1000μL)
- Incubated at 30°C, measuring the fluorescent intensity intermittently
Sender; LuxI plasmid transformed into E.coli strains (JW1908) Receiver; LuxR-gfp plasmid transformed into E.coli strains (JW1908) Culture/ Cndn.
# Both Sender and Receiver (+/- Mutation) were inoculated into small (2mL) culture and was shaken separately for 12h (at 37C) # Inoculated into flesh media, shaken until cell density hit 2.0 in OD600 # Washed the cell and re-suspended. Cell density checked. # Mixed Sender and Receiver (Sender/Receiver 1000μl/1000μl). # Incubated at 30°C. # Time-chased the fluorescence (485nm(excitation) and 527nm(emission)) by gfp.
|
|
| [http://partsregistry.org/Part:BBa_S03623 BBa_S03623] |
|
|
Reference
- Collins et.al. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005)
- B.Koch et.al.:The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors. Microbiol. 2005 Nov;151(Pt 11):3589-602.
|
|
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"