Team:NTU-Singapore/Project/Specification
From 2008.igem.org
(→Specification) |
(→Transformation of parts) |
||
| Line 57: | Line 57: | ||
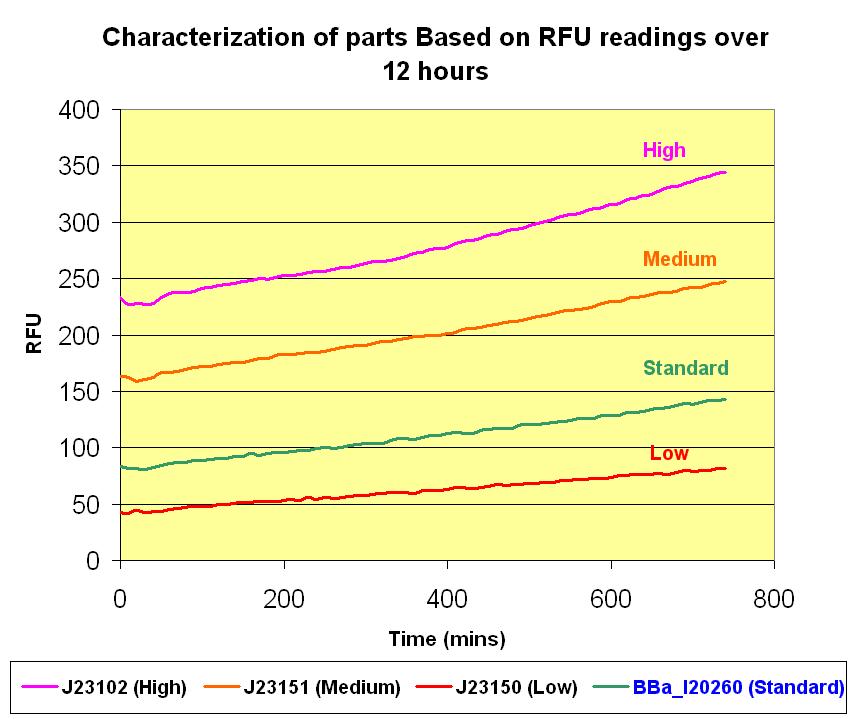
With the parts transformed, we can proceed with the characterization based on the protocol we constructed. We make use of the FLx800™ Fluorescence Microplate Reader to take the readings of the fluorescence that is emitted by the GFP reporting devices by measuring the relative fluorescence units (RFU). The different strength of the promoters associated with the same GFP reporting device, E0240 will determine the rate of transcription of the GFP protein. The stronger the promoter, the higher is the rate of its transcription. This results in more GFP proteins formed which in turns relates to more fluorescence being emitted. This direct proportionality brings us closer to quantifying the strength of any promoter of interest. Hence a standard promoter was used to compare the RFU with other promoters. Based on the newsletter, there are also the Low, Medium and High promoters. We transformed all these parts and take their readings over 12 hours at 10 minutes intervals. The graphs below illustrates the results we obtained and the detailed data points and graphs plotting can be retrieved from the excel file. | With the parts transformed, we can proceed with the characterization based on the protocol we constructed. We make use of the FLx800™ Fluorescence Microplate Reader to take the readings of the fluorescence that is emitted by the GFP reporting devices by measuring the relative fluorescence units (RFU). The different strength of the promoters associated with the same GFP reporting device, E0240 will determine the rate of transcription of the GFP protein. The stronger the promoter, the higher is the rate of its transcription. This results in more GFP proteins formed which in turns relates to more fluorescence being emitted. This direct proportionality brings us closer to quantifying the strength of any promoter of interest. Hence a standard promoter was used to compare the RFU with other promoters. Based on the newsletter, there are also the Low, Medium and High promoters. We transformed all these parts and take their readings over 12 hours at 10 minutes intervals. The graphs below illustrates the results we obtained and the detailed data points and graphs plotting can be retrieved from the excel file. | ||
| + | |||
| + | [[Image:NTU_Characterization_Graph_Standards_(12_hours).JPG]] | ||
| + | NTU_Characterization_Graph_Standards_(3.5_hours).JPG | ||
Revision as of 08:47, 26 July 2008
|
Contents |
Specification
Introduction
The group proposed to discuss and investigate the working range of the parts and devices of our system
- The proposed variables to investigate are:
i) Concentration of activator
ii) Time
iii) Temperature
Characterization of Parts
We are interested in characterizing the parts
i) Lactose/IPTG sensitive Promoter pLac [http://partsregistry.org/wiki/index.php?title=Part:BBa_R0010 BBa_R0010]
ii) Iron Sensitive Promoter pFe [http://partsregistry.org/wiki/index.php?title=Part:BBa_I716014 BBa_I716014]
The characterization is carried out by ligating the desired promoter with the Green Fluorescence protein: [http://partsregistry.org/Part:BBa_E0240 BBa_E02040]
- For the pLac BBa_R0010, it is already available in the form we desired in the registry as [http://partsregistry.org/wiki/index.php?title=Part:BBa_J04430 BBa_J04430]
The FLx800™ Fluorescence Microplate Reader
The FLx800™ Fluorescence Microplate Reader was available for our use courtesy of Division of Bio-engineering lab. It was purchased from BioTek and for more information about the reader, you can refer to [http://www.biotek.com/products/product_detail.php?pid=117 The FLx800™ Fluorescence Microplate Reader]
The FLx800™ Fluorescence Microplate Reader has a computer software KCJunior that was installed in a computer adjacent to the reader for collection and processing of data from our samples.
The Standardistation
In the first edition of the newsletter, it elaborated a method similar to the approach our characterization procedure. We have decided to make use of the protocol given to us, however with some minor modifications.
Transformation of parts
The parts transformed are:
- LacI-GFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_I763004 BBa_I763004],
- Standard promoter with GFP reporter [http://partsregistry.org/Part:BBa_I20260 BBa_I20260]
- GFP reporter devices with Weak promoter[http://partsregistry.org/Part:BBa_J23150 BBa_J23150],
- Medium promoter [http://partsregistry.org/Part:BBa_J23151 BBa_J23151] and
- Strong promoter [http://partsregistry.org/Part:BBa_J23102 BBa_J23102]
With the parts transformed, we can proceed with the characterization based on the protocol we constructed. We make use of the FLx800™ Fluorescence Microplate Reader to take the readings of the fluorescence that is emitted by the GFP reporting devices by measuring the relative fluorescence units (RFU). The different strength of the promoters associated with the same GFP reporting device, E0240 will determine the rate of transcription of the GFP protein. The stronger the promoter, the higher is the rate of its transcription. This results in more GFP proteins formed which in turns relates to more fluorescence being emitted. This direct proportionality brings us closer to quantifying the strength of any promoter of interest. Hence a standard promoter was used to compare the RFU with other promoters. Based on the newsletter, there are also the Low, Medium and High promoters. We transformed all these parts and take their readings over 12 hours at 10 minutes intervals. The graphs below illustrates the results we obtained and the detailed data points and graphs plotting can be retrieved from the excel file.
NTU_Characterization_Graph_Standards_(3.5_hours).JPG "
"