Team:BCCS-Bristol/Protocols-Agarose Gel Electrophoresis
From 2008.igem.org
(Difference between revisions)
Tgorochowski (Talk | contribs) |
Tgorochowski (Talk | contribs) |
||
| Line 26: | Line 26: | ||
# Pour the gel into a chamber with a comb that you prepared before | # Pour the gel into a chamber with a comb that you prepared before | ||
# Let the gel cool down and become solid (15-20 min) | # Let the gel cool down and become solid (15-20 min) | ||
| - | |||
===Sample preparation: === | ===Sample preparation: === | ||
| Line 32: | Line 31: | ||
# Add 10 % “Sample Loading Buffer” (BIORAD) to the sample | # Add 10 % “Sample Loading Buffer” (BIORAD) to the sample | ||
# Mix gently and spin shortly down | # Mix gently and spin shortly down | ||
| - | |||
===Loading the gel: === | ===Loading the gel: === | ||
| Line 42: | Line 40: | ||
# Close the chamber with the lid (black pole to black=negatively charged and red to red=positively charged…) | # Close the chamber with the lid (black pole to black=negatively charged and red to red=positively charged…) | ||
# Run the gel with 80-100 V | # Run the gel with 80-100 V | ||
| - | |||
The Sample Loading Buffer contains two dyes: Bromophenol blue runs at ~300 bp and Xylene cyanol FF runs at ~4 kb. | The Sample Loading Buffer contains two dyes: Bromophenol blue runs at ~300 bp and Xylene cyanol FF runs at ~4 kb. | ||
Revision as of 19:53, 12 August 2008
Agarose Gel Electrophoresis
Gel preparation:
- For a thick gel in a small chamber, boil 50 ml 0.5x TBE in the microwave (for a thinner gel to insert only 5 µl sample in each well 40 ml are sufficient)
- Cool the solution until you can touch it with your hands for a longer time
- Add 0.5 µl ethidium bromide per 10 ml TBE and mix while avoiding air bubbles
- Pour the gel into a chamber with a comb that you prepared before
- Let the gel cool down and become solid (15-20 min)
Sample preparation:
- Add 10 % “Sample Loading Buffer” (BIORAD) to the sample
- Mix gently and spin shortly down
Loading the gel:
- Remove the comb and put the gel with the slide into a chamber (the wells need to be on the end with the black pole!!!)
- Fill the chamber with 0.5x TBE until the gel is covered
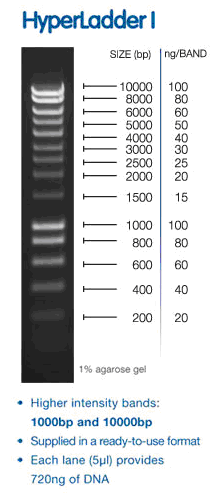
- Use 5 µl HyperLadderI (BIOLINE)
- For colony PCR, put 5 µl of each reaction in the well
- Close the chamber with the lid (black pole to black=negatively charged and red to red=positively charged…)
- Run the gel with 80-100 V
The Sample Loading Buffer contains two dyes: Bromophenol blue runs at ~300 bp and Xylene cyanol FF runs at ~4 kb.
|
 "
"