Imperial College/5 September 2008
From 2008.igem.org
m |
|||
| Line 35: | Line 35: | ||
===Suggestions for Further Improvements=== | ===Suggestions for Further Improvements=== | ||
| - | Pipette tips should be changed... | + | Pipette tips should be changed after each transfer of solution during serial dilution. This would avoid contamination and produce more accurate results for the calibration curve. |
| + | |||
| + | Choose dilution factors for plating more carefully. It is not advisable to choose very high dilution factors e.g. those higher than 10E8. | ||
{{Imperial/EndPage}} | {{Imperial/EndPage}} | ||
Revision as of 17:27, 5 September 2008
5 September 2008WetlabCloning
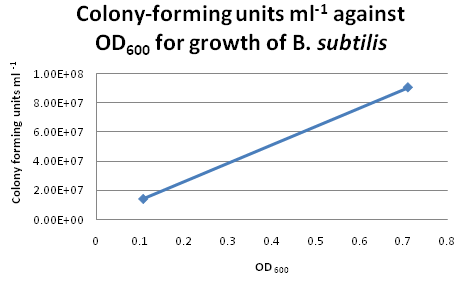
Colony CountingAfter re-calibrating our illuminator, it can now count colonies with a fair degree of accuracy. Manual addition and removal of points allows us to fine-tune the results, so we will use this method from now on. Trial Run: B. subtilis Growth CurveIn our second trial run to obtain growth and calibration curves for B. subtilis, we cultured our "B. subtilis" for two days and continously monitored the changes in OD600. By plotting OD600 against Time/h, we obtained the following growth curve for "B. subtilis". Calibration CurveFor six of the data points from the above growth curve, we plated 0.1 ml of B. subtilis on LB agar plates and performed serial dilution in order to obtain the colony-forming units (CFU per ml) for the corresponding OD600. However, we only managed to obtain two sets of valid data. The B. subtilis mixtures we plated for higher values of OD600 were too dilute, hence no bacterial growth were observed on these LB agar plates. Suggestions for Further ImprovementsPipette tips should be changed after each transfer of solution during serial dilution. This would avoid contamination and produce more accurate results for the calibration curve. Choose dilution factors for plating more carefully. It is not advisable to choose very high dilution factors e.g. those higher than 10E8.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"