Team:Caltech/Protocols/Folate assay
From 2008.igem.org
(→L. rhamnosus inoculum preparation) |
(→Sample Preparation) |
||
| Line 53: | Line 53: | ||
===Sample Preparation=== | ===Sample Preparation=== | ||
| - | + | # Centrifuge the full-grown cell culture (5ml) after centrifugation (13,000x g, 10 min, 20 C). | |
| - | + | # Recover both cells and supernatant. | |
| - | + | # Dilute the supernatant 1:1 with 0.1M sodium acetate buffer (pH4.8) -1% ascorbic acid. | |
| - | + | # Wash with the 0.1M sodium acetate-1% ascorbic acid and resuspend in 5 mL of the same buffer. | |
| - | + | # Release folate from the cells by incubating the samples at 100C for 5 min (determined to be optimal for folate release + the heat inactivates the bacteria) | |
| - | + | # Add the deconjugation reaction mixture (2.5% vol/vol) | |
| - | + | # Incubate for 4h at 37C, you should see obvious cell debris at the bottom of the cell lysate tubes. | |
| - | + | # Filter sterilize 2.5 mL of each sample into a clean glass tube. | |
| - | + | # Add 2.5 mL of folate assay media to each tube, bringing the volume up to 5mL. | |
| - | + | # Add 50uL of prepared ''L.rhamnosus'' inoculum to each assay tube. | |
| - | + | # Incubate for 16-20 hours at 37C. | |
| - | + | # Read the absorbances of the samples at 546 nm on a plate reader (200uL per well). The protocol recommends refrigerating the samples at 4C for 15-30 minutes prior to reading, but I don't think it makes a difference, particularly if you intend on returning the samples to the incubator to take later timepoints. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Standard Curve=== | ===Standard Curve=== | ||
Revision as of 21:18, 6 September 2008
|
People
|
{{{Content}}} |
</div>
Folate Microbiological Assay ProtocolPurposeTo quantify and measure levels of folate production in the cell lysate and supernatant of 'E.coli' transformed with high copy folate biosynthesis genes folB, folKE, folBKE. The assay uses growth of the folate-dependent strain L. rhamnosus as an indicator of folate concentrations in the sample. Growth is measured by taking the OD at 546nm. Important Notes
Materials
Folic Acid Assay Media
Deconjugation Mixture
L. rhamnosus inoculum preparation
Sample Preparation
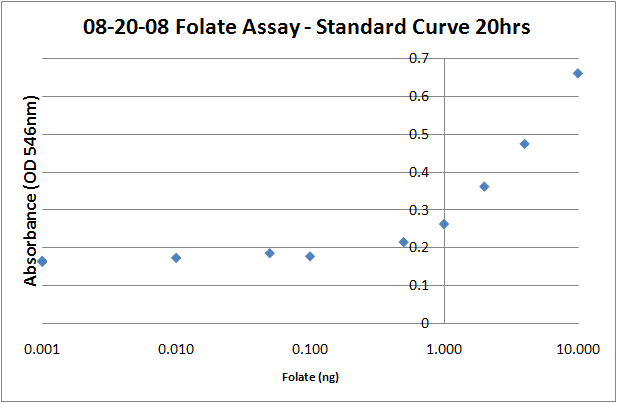
Standard Curve1. Prepare folic acid dilutions from 0- 10 ng/mL. Usually we used 0.001, 0.01, 0.05, 0.1, 0.5, 1, 2, 4, 8, 10ng samples. With L. rhamnosus the linear range of the assay appears to be from 0.1 to 10 ng. 2. Add 2.5 mL of folic acid assay media to each tube, along with 2.5 mL folic acid + water to make up a total volume of 5mL. 3. Add 50uL of the prepared L. rhamnosus inoculum. 4. Incubate for 16-20 hours at 37C. 5. Read the absorbances of the samples at 546 nm on a plate reader (200uL per well). The protocol recommends refrigerating the samples at 4C for 15-30 minutes prior to reading, but I don't think it makes a difference, particularly if you intend on returning the samples to the incubator to take later timepoints. Sources
|
 "
"