Team:University of Lethbridge/Notebook/GeneralLabJune
From 2008.igem.org
(→June 6 2008) |
Munima.alam (Talk | contribs) (spellcheck) |
||
| Line 8: | Line 8: | ||
Stored media in fridge. | Stored media in fridge. | ||
| + | |||
===June 10 2008=== | ===June 10 2008=== | ||
====Christa, Munima, Roxanne, and Sebastian==== | ====Christa, Munima, Roxanne, and Sebastian==== | ||
Prepared 1L of liquid media following the procedure found on OpenWetWare. | Prepared 1L of liquid media following the procedure found on OpenWetWare. | ||
| - | -10g peptone | + | -10g peptone (substituted for tryptone) |
-10g NaCl | -10g NaCl | ||
-5g Yeast Extract | -5g Yeast Extract | ||
| Line 25: | Line 26: | ||
====Roxanne==== | ====Roxanne==== | ||
Defrosted the -20 freezer in the teaching lab for iGEM use (with a little help from my friends!) | Defrosted the -20 freezer in the teaching lab for iGEM use (with a little help from my friends!) | ||
| + | |||
===June 11 2008=== | ===June 11 2008=== | ||
====Sebastian, Munima, Roxanne, Christa==== | ====Sebastian, Munima, Roxanne, Christa==== | ||
| - | Poured 8 minimal media (labeled control- with blue sharpie) plates and 14 amp plates (labeled amp - with red sharpie) | + | Poured 8 minimal media (labeled control - with blue sharpie) plates and 14 amp plates (labeled amp - with red sharpie) |
stored in the 4 C fridge. Amp concentration is always 50ug/mL. | stored in the 4 C fridge. Amp concentration is always 50ug/mL. | ||
| + | |||
===June 16 2008=== | ===June 16 2008=== | ||
| Line 36: | Line 39: | ||
Plated cheZ knockout strain from glycerol on LB + amp and Plain LB to assess viability and antibiotic resistance at 37 C overnight. | Plated cheZ knockout strain from glycerol on LB + amp and Plain LB to assess viability and antibiotic resistance at 37 C overnight. | ||
| - | Transformed | + | Transformed supercompetent cells with basic biobrick vector pSB1A7 (ampicillin resistance) |
-50 uL of cells, 0.85 uL 2-mercaptoethanol, 1 uL of plasmid dissolved with 15 uL of ddH2O | -50 uL of cells, 0.85 uL 2-mercaptoethanol, 1 uL of plasmid dissolved with 15 uL of ddH2O | ||
-30 min on ice | -30 min on ice | ||
| Line 42: | Line 45: | ||
-2 min on ice | -2 min on ice | ||
-Add 0.9 mL of LB shaker incubate at 225 RPM and 37 C | -Add 0.9 mL of LB shaker incubate at 225 RPM and 37 C | ||
| + | |||
===June 17 2008=== | ===June 17 2008=== | ||
====Munima, Christa, Nathan Puhl==== | ====Munima, Christa, Nathan Puhl==== | ||
Checked plates | Checked plates | ||
| - | -cheZ knockout strain viable on | + | -cheZ knockout strain viable on LB, no growth on LB + amp - Good |
-Only one colony from transformation, not very good efficiency, don't know why because too many possibilities, most | -Only one colony from transformation, not very good efficiency, don't know why because too many possibilities, most | ||
| - | likely amount of DNA due to inability to | + | likely amount of DNA due to inability to quantify plasmid from iGEM plates |
-Subcultured colony in liquid LB + amp | -Subcultured colony in liquid LB + amp | ||
-Plate 200 uL on LB + amp at 37 C overnight | -Plate 200 uL on LB + amp at 37 C overnight | ||
| Line 57: | Line 61: | ||
Made glycerol stock of pSB1A7 transformed E. coli | Made glycerol stock of pSB1A7 transformed E. coli | ||
| - | Extracted plasmid from transformed E. coli using the | + | Extracted plasmid from transformed E. coli using the Eppendorf FastPlasmid minikit and stored 4 aliquots of 25 uL in the -20 C freezer. |
| + | |||
===June 19 2008=== | ===June 19 2008=== | ||
Revision as of 19:05, 24 June 2008
Contents |
June 6 2008
Sebastian, John and Roxanne
Prepared 1L of semi-solid media following the procedure found on OpenWetWare.
-10g peptone (substituted for tryptone) -10g Agar -10g NaCl -5g Yeast Extract
Stored media in fridge.
June 10 2008
Christa, Munima, Roxanne, and Sebastian
Prepared 1L of liquid media following the procedure found on OpenWetWare.
-10g peptone (substituted for tryptone) -10g NaCl -5g Yeast Extract
Poured 36 culture test tubes, and the remainder was left in a 1L Erlenmeyer flask. Stored both in fridge.
Christa
Made an inventory of iGEM 2007 parts in Wieden -80 freezer inventory.xls
Roxanne
Defrosted the -20 freezer in the teaching lab for iGEM use (with a little help from my friends!)
June 11 2008
Sebastian, Munima, Roxanne, Christa
Poured 8 minimal media (labeled control - with blue sharpie) plates and 14 amp plates (labeled amp - with red sharpie) stored in the 4 C fridge. Amp concentration is always 50ug/mL.
June 16 2008
Nathan Puhl, Munima, Christa, Sebastian, Roxanne
Plated cheZ knockout strain from glycerol on LB + amp and Plain LB to assess viability and antibiotic resistance at 37 C overnight.
Transformed supercompetent cells with basic biobrick vector pSB1A7 (ampicillin resistance)
-50 uL of cells, 0.85 uL 2-mercaptoethanol, 1 uL of plasmid dissolved with 15 uL of ddH2O -30 min on ice -45 s at 42 C -2 min on ice -Add 0.9 mL of LB shaker incubate at 225 RPM and 37 C
June 17 2008
Munima, Christa, Nathan Puhl
Checked plates
-cheZ knockout strain viable on LB, no growth on LB + amp - Good -Only one colony from transformation, not very good efficiency, don't know why because too many possibilities, most likely amount of DNA due to inability to quantify plasmid from iGEM plates -Subcultured colony in liquid LB + amp -Plate 200 uL on LB + amp at 37 C overnight
June 18 2008
Munima, Christa, Alix, Nathan Puhl
Made glycerol stock of pSB1A7 transformed E. coli
Extracted plasmid from transformed E. coli using the Eppendorf FastPlasmid minikit and stored 4 aliquots of 25 uL in the -20 C freezer.
June 19 2008
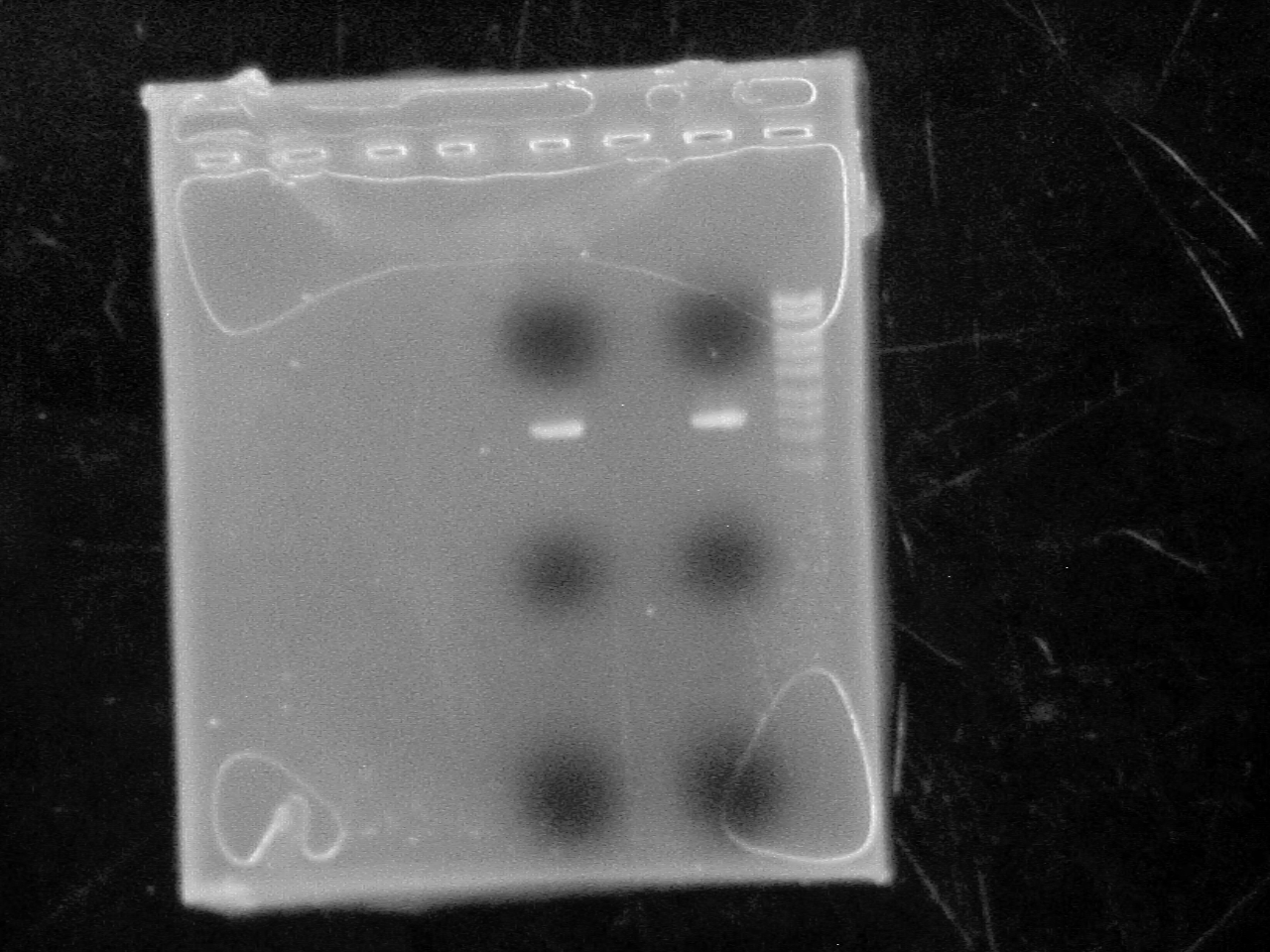
Nathan Puhl, Alix, Munima, Christa, Roxanne
Ran plasmids on 1% agarose gel with High range ladder
 "
"