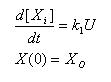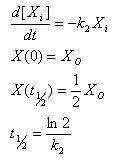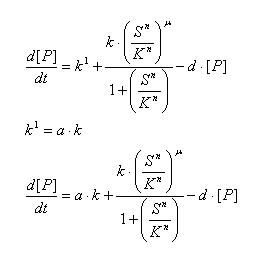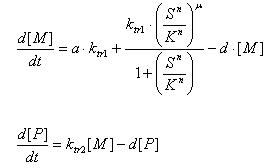Team:NTU-Singapore/Modelling/ODE
From 2008.igem.org
Lalala8585 (Talk | contribs) (New page: <html><link rel="stylesheet" href="http://greenbear88.googlepages.com/ntu_igem.css" type="text/css"></html> <div id="header">{{User:Greenbear/sandbox/header}}</div> <div id="maincontent"...) |
Lalala8585 (Talk | contribs) |
||
| Line 11: | Line 11: | ||
==Constant synthesis & Linear Synthesis== | ==Constant synthesis & Linear Synthesis== | ||
| + | [[Image:Linear_synthesis.JPG |100px|Linear Synthesis]] | ||
| + | |||
*Simple ode to describe constant synthesis | *Simple ode to describe constant synthesis | ||
*Gives an explicit analytical solution | *Gives an explicit analytical solution | ||
| Line 16: | Line 18: | ||
==Linear Degradation== | ==Linear Degradation== | ||
| + | [[Image:Linear_degradation.JPG |100px|Linear Degradation]] | ||
| + | |||
*Rate of degradation is proportional to how much of the molecule is present | *Rate of degradation is proportional to how much of the molecule is present | ||
*Gives an explicit analytical solution | *Gives an explicit analytical solution | ||
| Line 21: | Line 25: | ||
==Simple Forward Reaction== | ==Simple Forward Reaction== | ||
| + | [[Image:Complex_formation.JPG |200px|Complex Formation]]<br> | ||
| + | |||
| + | [C] : Complex <br> | ||
| + | kc : Rate constant of complex formation<br> | ||
| + | |||
This equation ignores the fact that dissociation of the complex occurs. | This equation ignores the fact that dissociation of the complex occurs. | ||
We can do so if the dissociation is much slower than the formation. | We can do so if the dissociation is much slower than the formation. | ||
| Line 30: | Line 39: | ||
Assumptions: | Assumptions: | ||
*Linear kintic rate laws apply only if XT is much less than the Michaelis constants of both kinase and phosphotase | *Linear kintic rate laws apply only if XT is much less than the Michaelis constants of both kinase and phosphotase | ||
| + | [[Image:Phosphorylation.JPG|200px|Phosphorylation and Dephosphorylation]]<br> | ||
| + | XT : total cost of X protein in phosphorylated and unphosphorylated form<br> | ||
| + | S : protein kinase concentration<br> | ||
| + | k2 : accounts for protein phosphotase<br> | ||
*Modeled after simple linear kinetics | *Modeled after simple linear kinetics | ||
| Line 36: | Line 49: | ||
==Regulated Transcription== | ==Regulated Transcription== | ||
| + | |||
| + | [[Image:Regulated1.JPG|200px|Regulated1]]<br> | ||
| + | |||
| + | [P]: Protein Formed<br> | ||
| + | µ: Repression, µ=0;<br> Activation, µ=1<br> | ||
| + | K: Hill Constant Value of input that gives 50% response<br> | ||
| + | n: Hill coefficient Slope of signal-response curve at this input signal<br> | ||
| + | d: degradation of protein<br> | ||
| + | k1: basal gene expression<br> | ||
| + | k: signal-dependent gene expression<br> | ||
| + | a: correlation between k1 and k, 0<a<1<br> | ||
| + | |||
| + | [[Image:Regulated2.JPG|220px|Regulated2]]<br> | ||
| + | This ODE attempts to capture characteristics of the mRNA dynamics<br> | ||
| + | For our modeling, all our detection systems activates some form of transcription. Therefore µ=1 in all cases for our modeling exercise. | ||
| + | [[Image:Regulated3.JPG|220px|Regulated3]]<br> | ||
Revision as of 01:20, 26 June 2008
|
Contents |
ODEs used in modeling
The following equations shows the break down of the different equations that will be used in this modeling exercise. By understanding this section, it would make the understanding of the system of ODEs used
Constant synthesis & Linear Synthesis
- Simple ode to describe constant synthesis
- Gives an explicit analytical solution
- Unique solution once a IC is posed
Linear Degradation
- Rate of degradation is proportional to how much of the molecule is present
- Gives an explicit analytical solution
- Constant half life
Simple Forward Reaction
[C] : Complex
kc : Rate constant of complex formation
This equation ignores the fact that dissociation of the complex occurs. We can do so if the dissociation is much slower than the formation.
- Single solvable equation for the unknown C
- Simple, unique solution available with I.C
Phosphorylation and Dephosphorylation
Assumptions:
- Linear kintic rate laws apply only if XT is much less than the Michaelis constants of both kinase and phosphotase
XT : total cost of X protein in phosphorylated and unphosphorylated form
S : protein kinase concentration
k2 : accounts for protein phosphotase
- Modeled after simple linear kinetics
- Gives a hyperbolic signal response curve when X plotted vs S
Regulated Transcription
[P]: Protein Formed
µ: Repression, µ=0;
Activation, µ=1
K: Hill Constant Value of input that gives 50% response
n: Hill coefficient Slope of signal-response curve at this input signal
d: degradation of protein
k1: basal gene expression
k: signal-dependent gene expression
a: correlation between k1 and k, 0<a<1
This ODE attempts to capture characteristics of the mRNA dynamics
For our modeling, all our detection systems activates some form of transcription. Therefore µ=1 in all cases for our modeling exercise.
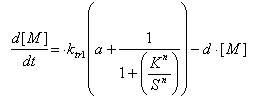
 "
"