Team:Chiba/Demo experiments
From 2008.igem.org
(→result and discussion-いろんなレシーバー実験) |
|||
| Line 1: | Line 1: | ||
| - | <html><link rel="stylesheet" href="https://2008.igem.org/wiki/index.php?title=User:Hiroki/style.css&action=raw&ctype=text/css" type="text/css" /></html> | + | |
| + | <html><link rel="stylesheet" | ||
| + | href="https://2008.igem.org/wiki/index.php?title=User:Hiroki/style.css&action=raw&ctype=text/css" | ||
| + | type="text/css" /></html> | ||
[[Image:Chiba-U.gif]] | [[Image:Chiba-U.gif]] | ||
| - | {| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | + | {| style="color:white;background-color:Maroon" cellpadding="3" |
| + | cellspacing="3" border="1" bordercolor="white" width="100%" | ||
| + | align="center" | ||
!align="center"|[[Team:Chiba|Home]] | !align="center"|[[Team:Chiba|Home]] | ||
!align="center"|[[Team:Chiba/Team|The Team]] | !align="center"|[[Team:Chiba/Team|The Team]] | ||
| Line 13: | Line 18: | ||
== Demo Experiment ~Senders~ == | == Demo Experiment ~Senders~ == | ||
===Method=== | ===Method=== | ||
| - | # | + | #Pre-culture |
| - | ## | + | ##Picked and cultured the following glycerol stocks in 2mL of LB: |
###LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (BW) | ###LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (BW) | ||
| - | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), (XL10G) | + | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 |
| - | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]), (XL10G) | + | BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 |
| - | ##37° | + | plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 |
| - | # | + | LuxI(no LVA)]), (XL10G) |
| - | ## | + | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007 |
| + | BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 | ||
| + | plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 | ||
| + | LasI(no LVA)]), (XL10G) | ||
| + | ##Cultured at 37°C for 12h. | ||
| + | #Culture | ||
| + | ##Added 6.25% each of the pre-cultures to new LB medium. | ||
###LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] | ###LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] | ||
| - | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]) | + | ###LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 |
| - | ##37° | + | BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 |
| + | plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 | ||
| + | LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 | ||
| + | BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 | ||
| + | plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 | ||
| + | LasI(no LVA)]) | ||
| + | ##Cultured at 37°C for 4~5h。 | ||
#Wash | #Wash | ||
| - | ## | + | ##Transfer 10mL each of the culture to 50mL centrifuge tubes. |
| - | ##20°C | + | ##Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant. |
| - | ## | + | ##Added LB-Amp to each centrifuge tube: |
| - | ###[http://partsregistry.org/Part:BBa_T9002 BBa_T9002] | + | ###10mL to the tube that contains |
| - | ###[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | + | [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] |
| - | ##[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | + | ###5mL to the tube that contains |
| - | ##[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | + | [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], |
| - | ##[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | + | [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] |
| - | ##[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | + | ##Centrifuged for 6min, 3600rpm at 20°C the tube containing |
| + | [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], | ||
| + | [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded | ||
| + | the supernatant. | ||
| + | ##10mL to the tube that contains | ||
| + | [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], | ||
| + | [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | ||
| + | ##Centrifuged for 6min, 3600rpm at 20°C the tube containing | ||
| + | [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], | ||
| + | [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded | ||
| + | the supernatant. | ||
| + | ##5mL to the tube that contains | ||
| + | [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], | ||
| + | [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] | ||
#Mix | #Mix | ||
| - | ## | + | ##Mixed the sender cells [http://partsregistry.org/Part:BBa_K084012 |
| - | ## | + | BBa_K084012] and [http://partsregistry.org/Part:BBa_K084007 |
| - | ### | + | BBa_K084007] both with [http://partsregistry.org/Part:BBa_T9002 |
| - | ### | + | BBa_T9002] at a 1:1 ratio. |
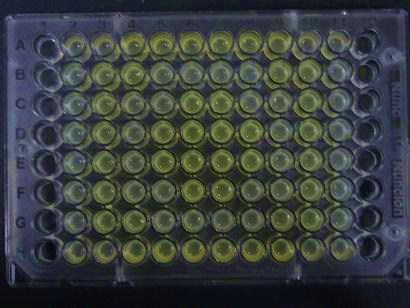
| - | ### | + | ##Added 100μL each to a 96-well shallow plate (as shown in the figure). |
| - | # | + | ###Green part is[http://partsregistry.org/Part:BBa_K084012 |
| + | BBa_K084012]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1 | ||
| + | ###Red part is [http://partsregistry.org/Part:BBa_K084007 | ||
| + | BBa_K084007]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1 | ||
| + | ###Uncolored part is [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] alone. | ||
| + | #Culture and observe results | ||
=== Results === | === Results === | ||
| - | [[Image:Team-Chiba-demo-mihon.gif|200px]] Green region: sender=LuxI, Red circular region: sender=Las I. | + | [[Image:Team-Chiba-demo-mihon.gif|200px]] Green region: sender=LuxI, |
| + | Red circular region: sender=Las I. | ||
<gallery> | <gallery> | ||
| Line 50: | Line 86: | ||
Image:Team-Chiba-demo-2.JPG|4 h <br>(Lux I GFP detected) | Image:Team-Chiba-demo-2.JPG|4 h <br>(Lux I GFP detected) | ||
Image:Team-Chiba-demo-3.JPG|8 h <br>(Lux I GFP and Las I GFP detected) | Image:Team-Chiba-demo-3.JPG|8 h <br>(Lux I GFP and Las I GFP detected) | ||
| - | </gallery> | + | </gallery> |
| - | + | LuxI GFP is detected at 4h following mixing while LasI GFP is detected | |
| + | after 8h, thus successfully demonstrating time-delay depending on the | ||
| + | sender used. | ||
| - | |||
--[[User:Yoshimi|Yoshimi]] 13:41, 29 October 2008 (UTC) | --[[User:Yoshimi|Yoshimi]] 13:41, 29 October 2008 (UTC) | ||
| Line 61: | Line 98: | ||
== Demo Experiment ~Receivers~ == | == Demo Experiment ~Receivers~ == | ||
| - | ===method | + | ===Varying bacterial numbers: method=== |
| - | #Receiver(T9002) | + | #Receiver(T9002) pre-incubation |
| - | ##Receiver:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW) | + | ##Receiver:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW)was |
| - | ## | + | cultured in 2mL LB-Amp (37°C,12h) |
| - | #Sender(S03623) | + | ##Pre-incubated Receiver([http://partsregistry.org/Part:BBa_T9002 |
| - | ##Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) | + | BBa_T9002](BW))was plated so as to produce about 1000 colonies. |
| - | # | + | #Sender(S03623) pre-incubation |
| - | ## | + | ##Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) was |
| - | ## | + | cultured in 50mL centrifuge tubes in 10mL of LB-Amp (37°C,12h)(2 |
| - | ## | + | tubes) |
| - | # | + | #Sender Wash |
| - | ##LB- | + | ##Centrifuged 2 tubes containing |
| - | ##LB- | + | ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW))at 20°C, |
| - | ##LB- | + | 3600rpm for 6min and discarded supernatant. |
| - | + | ##Added 10mL LB-Amp to each tube. | |
| - | # | + | ##Repeated wash twice. |
| - | ##Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW)) | + | #Creating bacterial plates |
| - | # | + | ##LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 |
| - | ##37° | + | BBa_S03623](BW)) tube 1 (10mL) was mixed with |
| + | LB-Amp-agar(50°C)(10ml)to produce sender containing bacterial | ||
| + | plate-1. | ||
| + | ##LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 | ||
| + | BBa_S03623](BW)) tube 2(100μl)was mixed with LB-Amp(9.9ml) and | ||
| + | diluted 100-fold. 10ml of this solution was mixed with | ||
| + | LB-Amp-agar(50°C)(10ml) and created | ||
| + | Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) | ||
| + | containing bacterial plate-2. | ||
| + | ##LB-Amp pre-cultured Sender solution-2(10μl) and LB-Amp(9.99ml) | ||
| + | was mixed to dilute 1000-fold。10ml of this solution and | ||
| + | LB-Amp-agar(50°C)(10ml) was mixed to create | ||
| + | Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) | ||
| + | containing bacterial plate-3 | ||
| + | #Lifted with nitrocellulose | ||
| + | ##Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW)) | ||
| + | colony was transfered to a nitrocellulose filter and placed on each of | ||
| + | Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) | ||
| + | containing bacterial plate (1~3) and Sender-absent negative control | ||
| + | plate (t=0). Determined the time required for the colonies to | ||
| + | fluoresce depending on the bacterial concentration (100 and 1000-fold | ||
| + | dilution). | ||
| + | #Method to detect fluorescence | ||
| + | ##Plates cultured at 37°C were exposed to UV (312nm) light once | ||
| + | every 30 minutes to observe GFP fluorescence. | ||
| Line 86: | Line 147: | ||
香取 | 香取 | ||
| - | === | + | ===Testing different receivers-methods=== |
| - | #Receiver& | + | #Receiver&sender pre-culture |
| - | ## | + | ##Used Receivers were: |
| - | + | ・[http://partsregistry.org/Part:BBa_T9002 | |
| + | BBa_T9002]:ptet-luxR-plux-GFP(high copy) | ||
| - | + | ・ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 | |
| + | BBa_J37032]:plux-GFP(high copy) | ||
| - | + | ・[http://partsregistry.org/Part:BBa_T9002 | |
| + | BBa_T9002]:ptet-luxR-plux-GFP(low copy) | ||
| - | + | ・ptet-mLuxR(too sensitive)-plux-GFP | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ・ptet-luxR-plux-GFP-plac-aiiA | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | (all BW) | |
| + | Each was cultured in 2ml LB (37°C,12h) and plated so that about | ||
| + | 1000 colonies of receiver cells will grow. | ||
| + | ##Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) was | ||
| + | cultured in 10mL LB in 50mL centrifuge tubes (37°C,12h) | ||
| + | #sender wash | ||
| + | ##Each receiver-containing medium was centrifuged in 50mL tubes at | ||
| + | de20°C, 3600rpm for 6min and supernatant discarded. | ||
| + | ##Added 10mL LB to each tube. | ||
| + | ##Repeated wash twice. | ||
| + | #Creating bacterial plates | ||
| + | ##LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 | ||
| + | BBa_S03623](BW)) tube 1 (10mL) was mixed with | ||
| + | LB-Amp-agar(50°C)(10ml)to produce sender containing bacterial | ||
| + | plate-1. | ||
| + | ##LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 | ||
| + | BBa_S03623](BW)) tube 2(100μl)was mixed with LB-Amp(9.9ml) and | ||
| + | diluted 100-fold. 10ml of this solution was mixed with | ||
| + | LB-Amp-agar(50°C)(10ml) and created | ||
| + | Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) | ||
| + | containing bacterial plate-2. | ||
| + | ##LB-Amp pre-cultured Sender solution-2(10μl) and LB-Amp(9.99ml) | ||
| + | was mixed to dilute 1000-fold。10ml of this solution and | ||
| + | LB-Amp-agar(50°C)(10ml) was mixed to create | ||
| + | Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) | ||
| + | containing bacterial plate-3 | ||
| + | #Lifted with nitrocellulose | ||
| + | ##Each Receiver colony was transfered to a nitrocellulose filter and | ||
| + | placed on a Sender([http://partsregistry.org/Part:BBa_S03623 | ||
| + | BBa_S03623](BW)) containing bacterial plate (1~3) or a sender-absent | ||
| + | negative control plate(t=0) to observe how receiver type affects the | ||
| + | time taken for the colonies to display visible fluorescence. | ||
| + | #Method to detect fluorescence | ||
| + | ##Plates cultured at 37°C were exposed to UV (312nm) light once | ||
| + | every 30 minutes to observe GFP fluorescence. | ||
| + | <BR> | ||
| + | |||
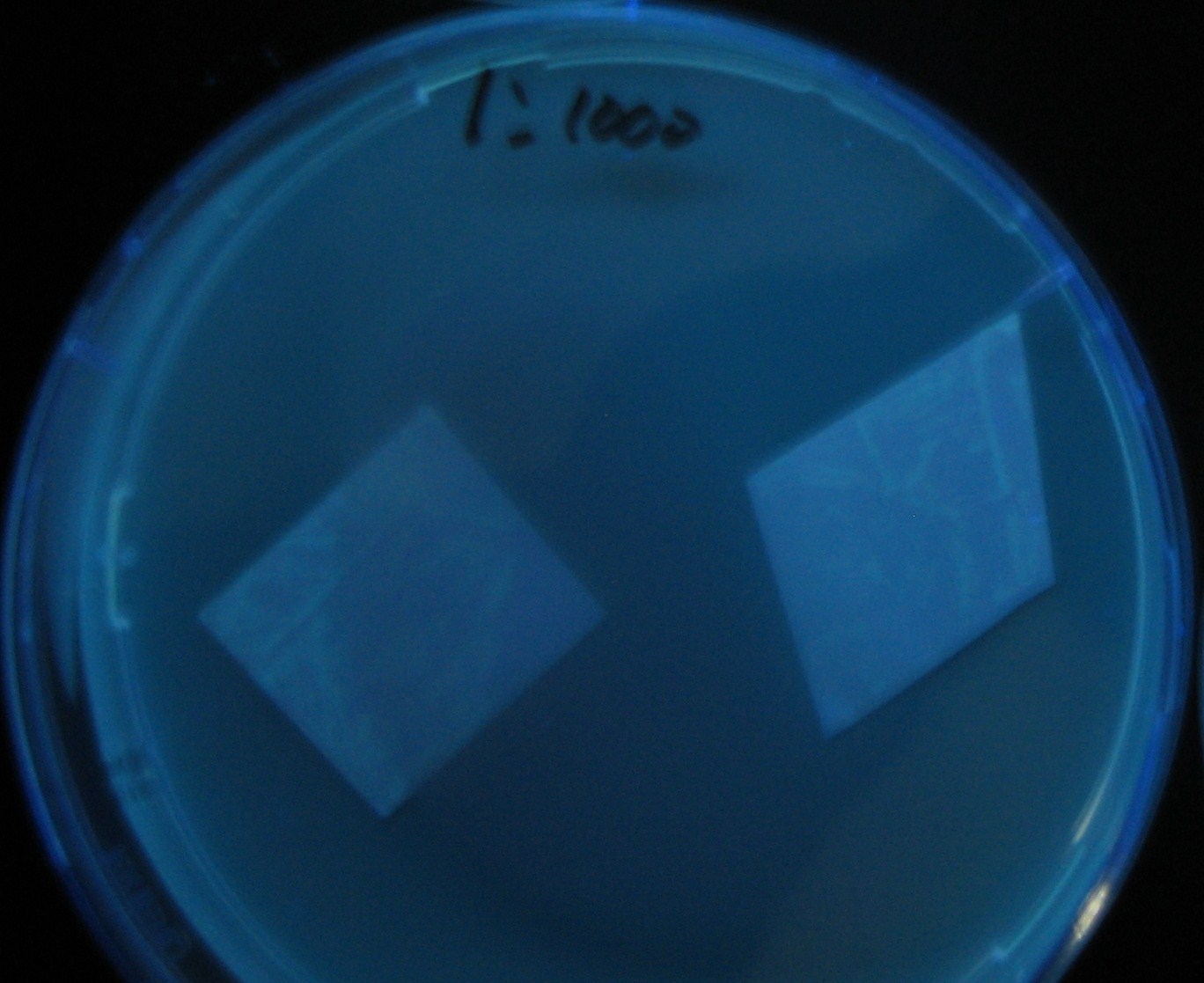
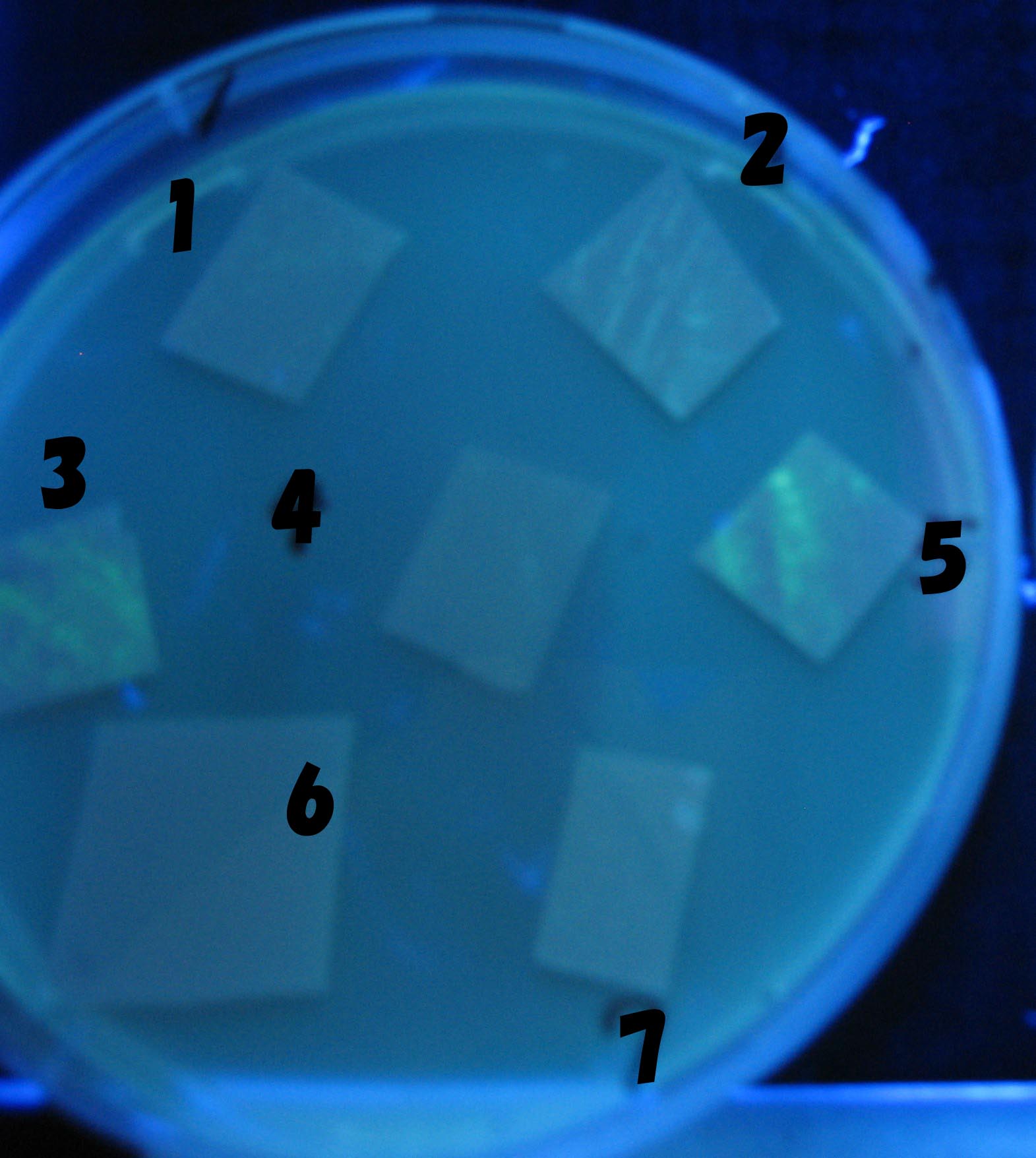
| + | ===Varying bacterial numbers-results=== | ||
| - | + | No Dilution | |
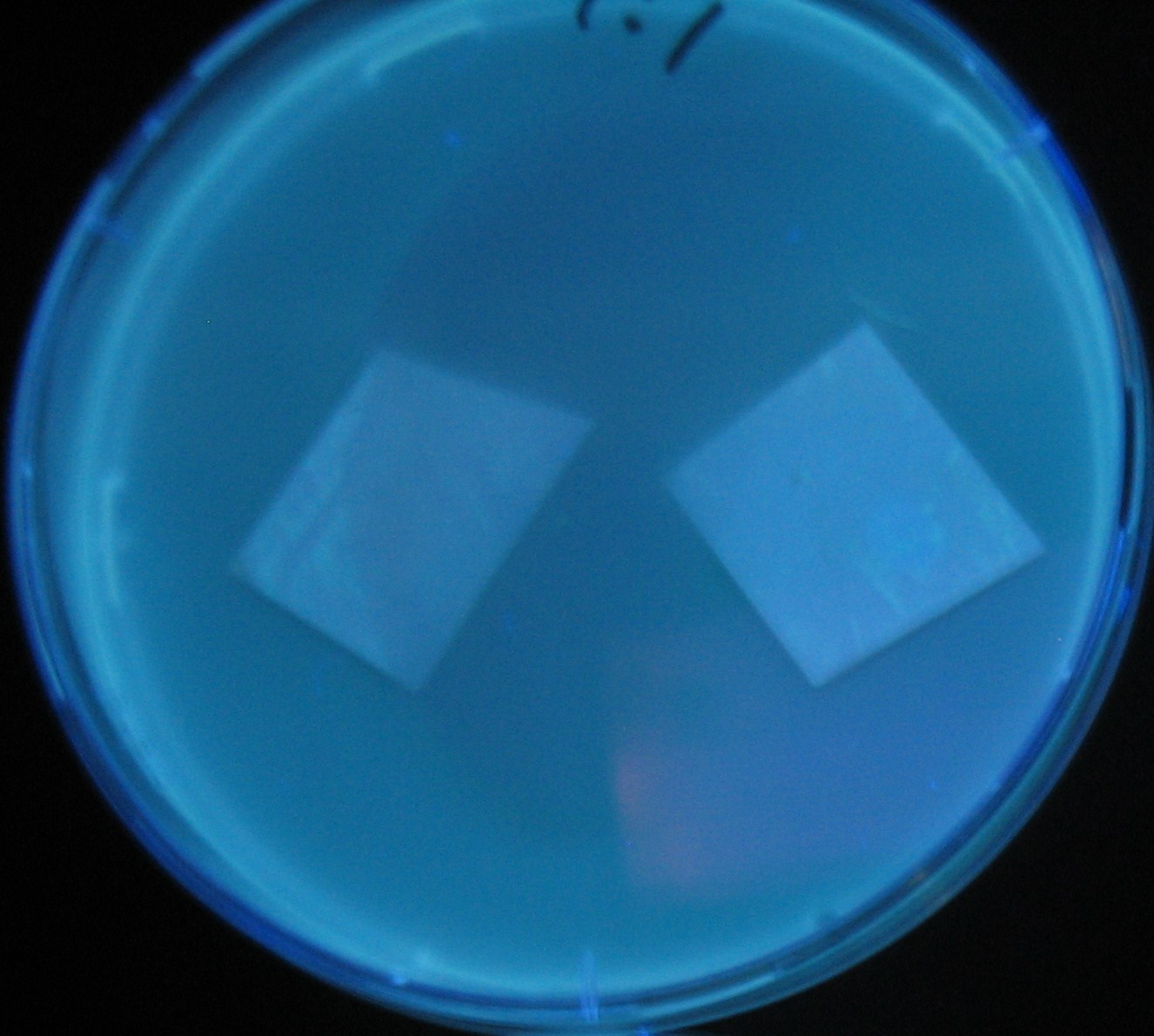
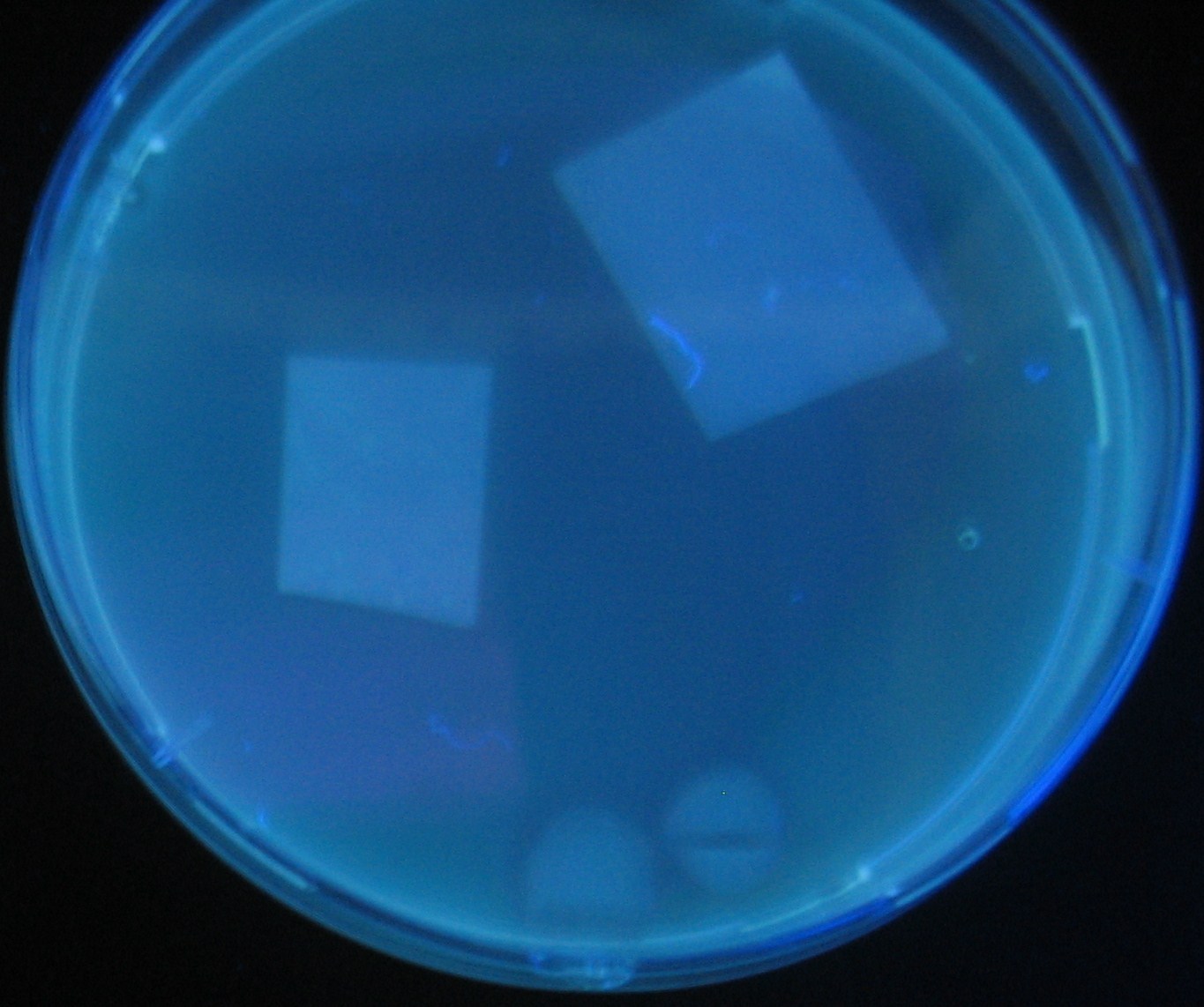
[[Image:Team-Chiba-IMG_0322-1.JPG|210px]] | [[Image:Team-Chiba-IMG_0322-1.JPG|210px]] | ||
[[Image:Team-Chiba-IMG_0331-1.JPG|225px]] | [[Image:Team-Chiba-IMG_0331-1.JPG|225px]] | ||
[[Image:Team-Chiba-IMG_0340.JPG|198px]] | [[Image:Team-Chiba-IMG_0340.JPG|198px]] | ||
| - | + | 0h 0.5h 1.5h | |
<BR> | <BR> | ||
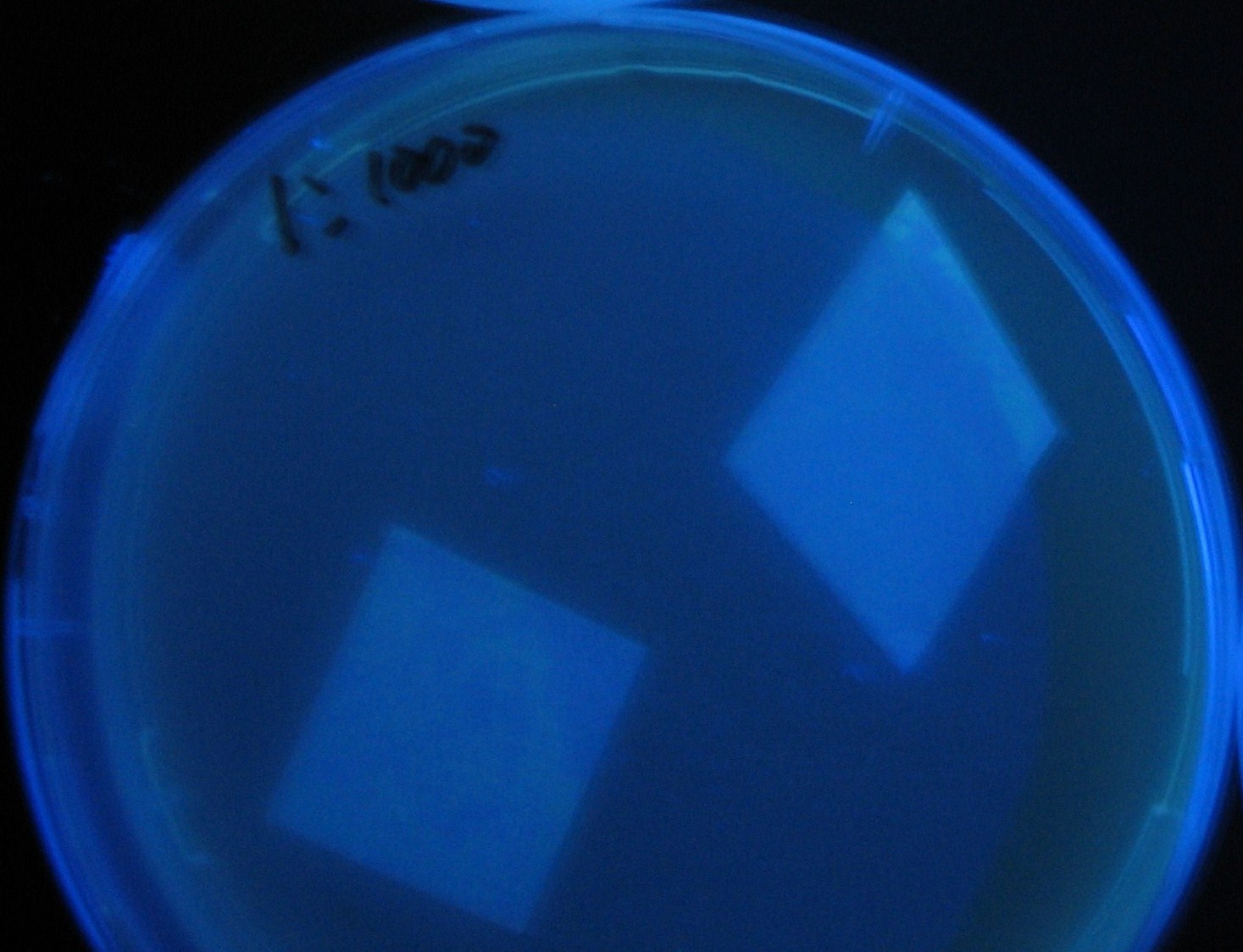
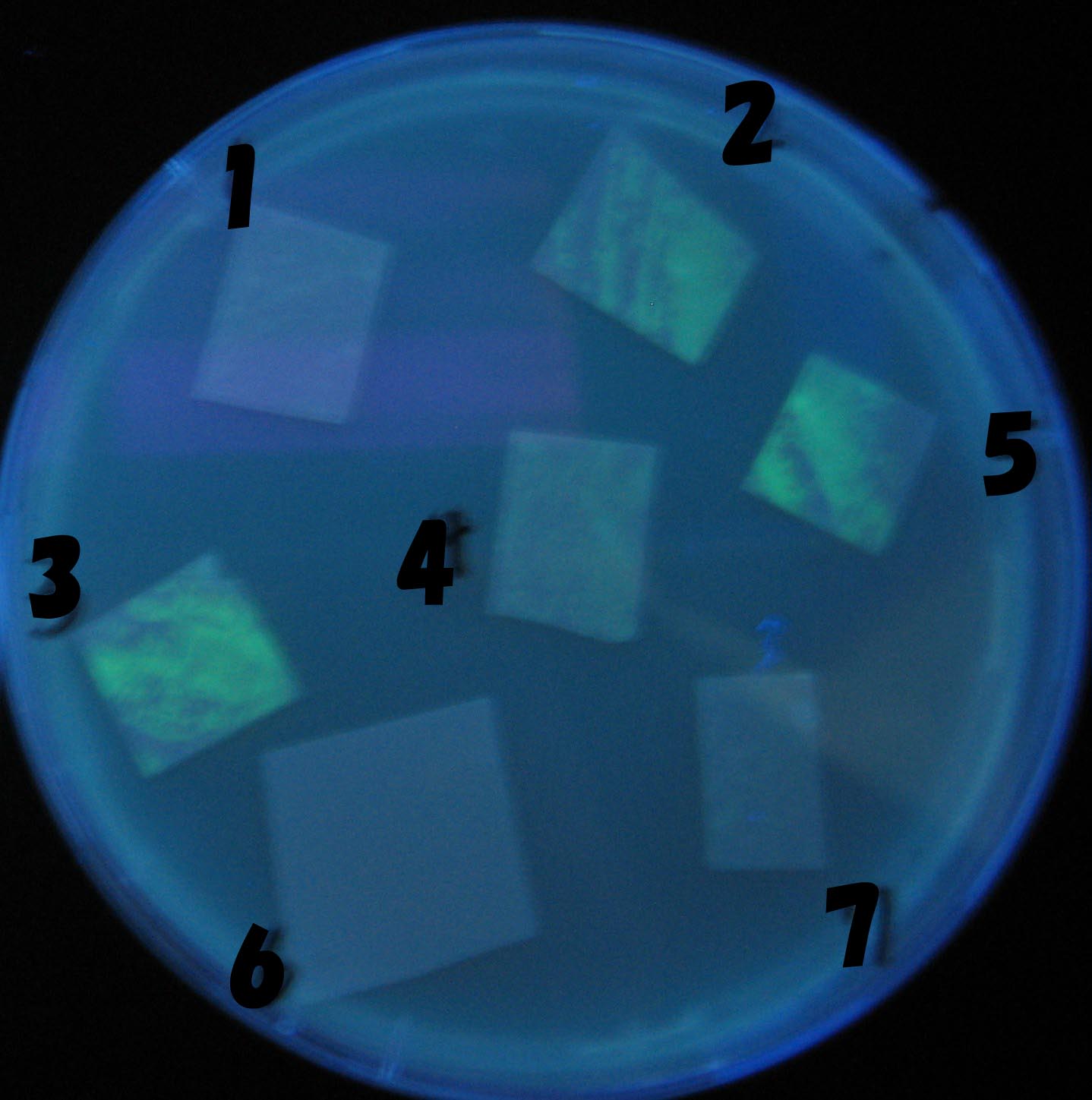
| - | + | 100-fold dilution | |
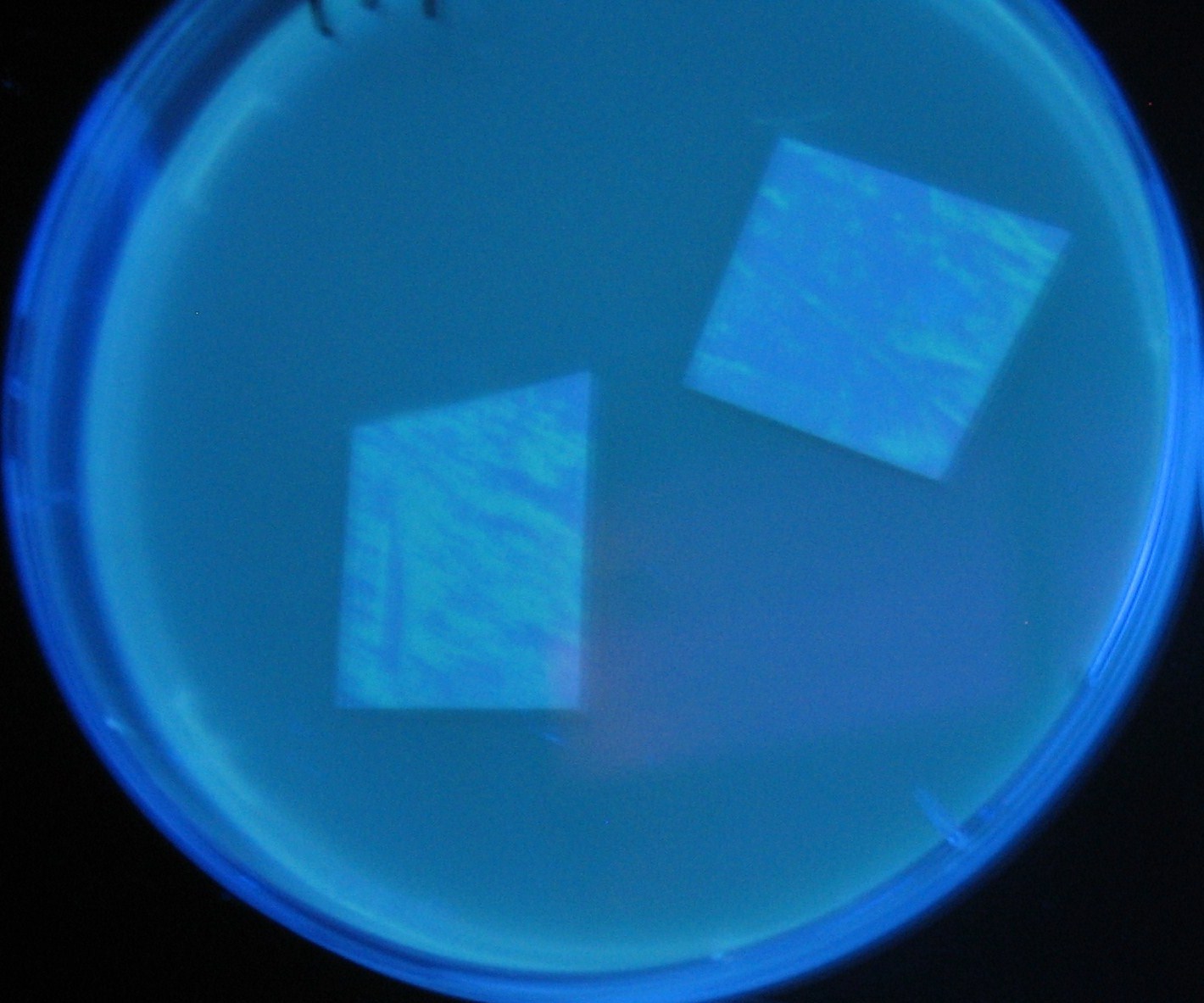
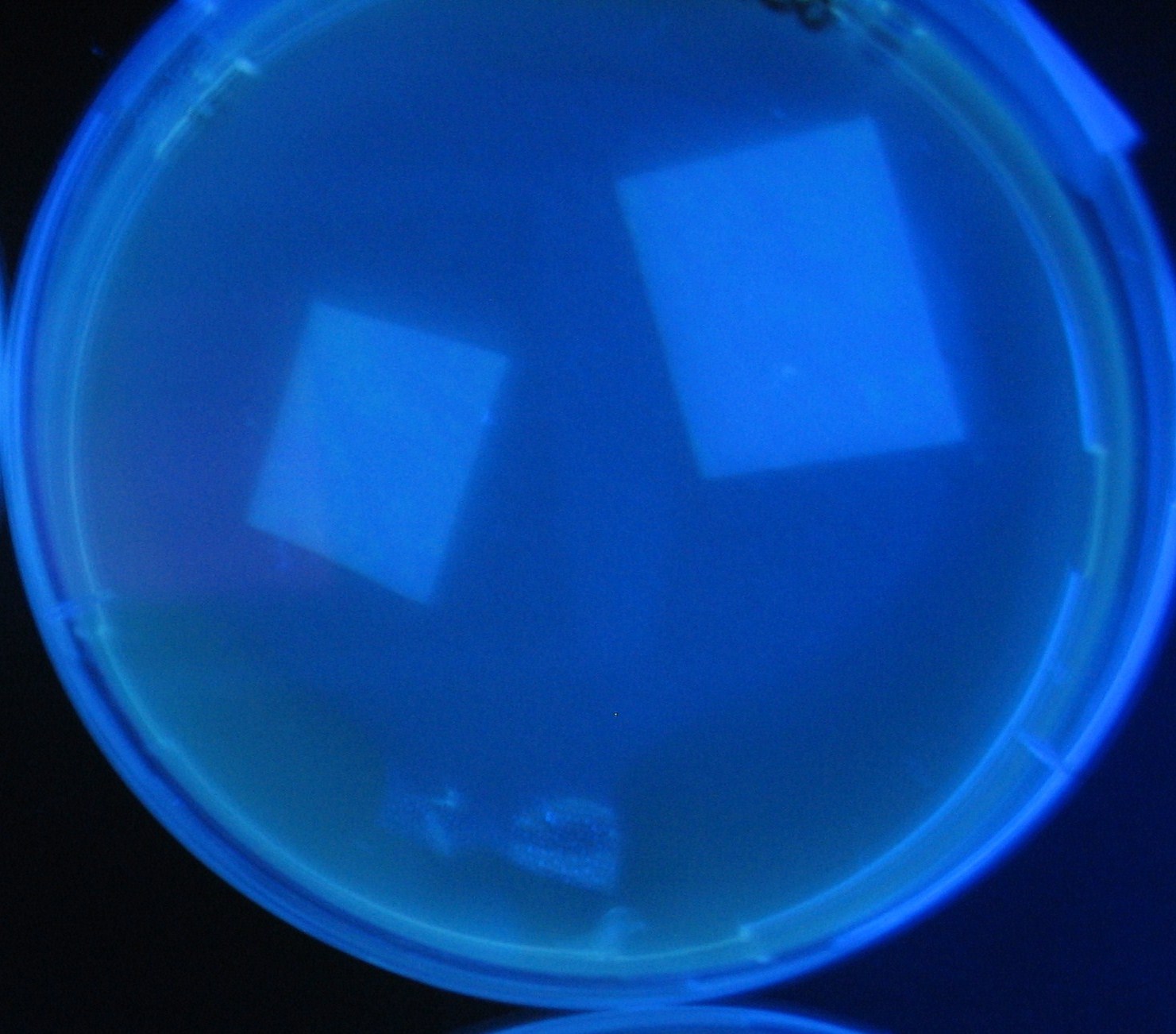
[[Image:Team-Chiba-IMG_0322-100.JPG|210px]] | [[Image:Team-Chiba-IMG_0322-100.JPG|210px]] | ||
[[Image:Team-Chiba-IMG_0331.JPG|200px]] | [[Image:Team-Chiba-IMG_0331.JPG|200px]] | ||
[[Image:Team-Chiba-IMG_0340-100.JPG|212px]] | [[Image:Team-Chiba-IMG_0340-100.JPG|212px]] | ||
[[Image:Team-Chiba-IMG_0349-100.JPG|179px]] | [[Image:Team-Chiba-IMG_0349-100.JPG|179px]] | ||
| - | <BR> | + | <BR> |
| - | + | 0h 0.5h | |
| + | 1.5h 2.0h | ||
<BR> | <BR> | ||
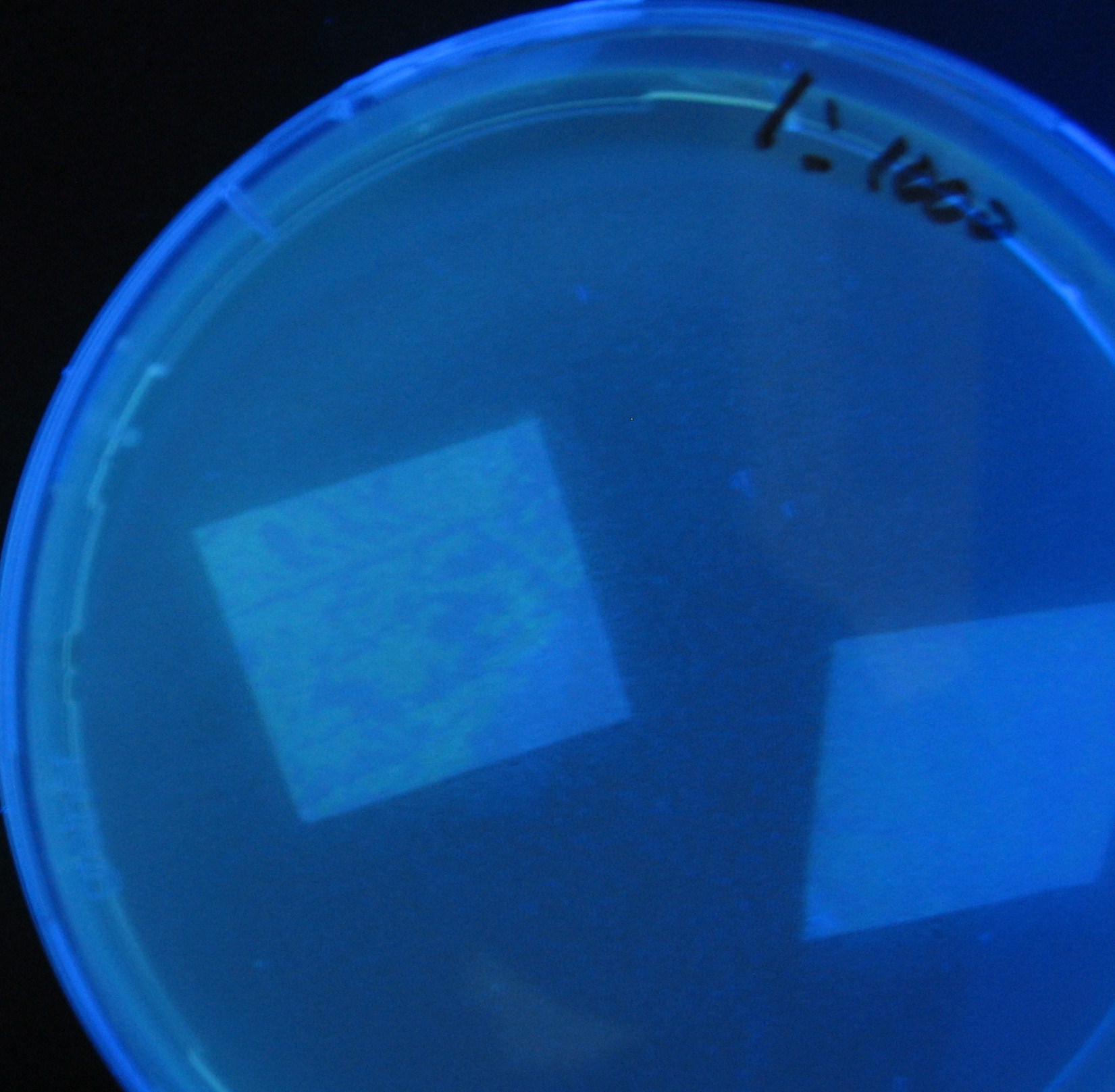
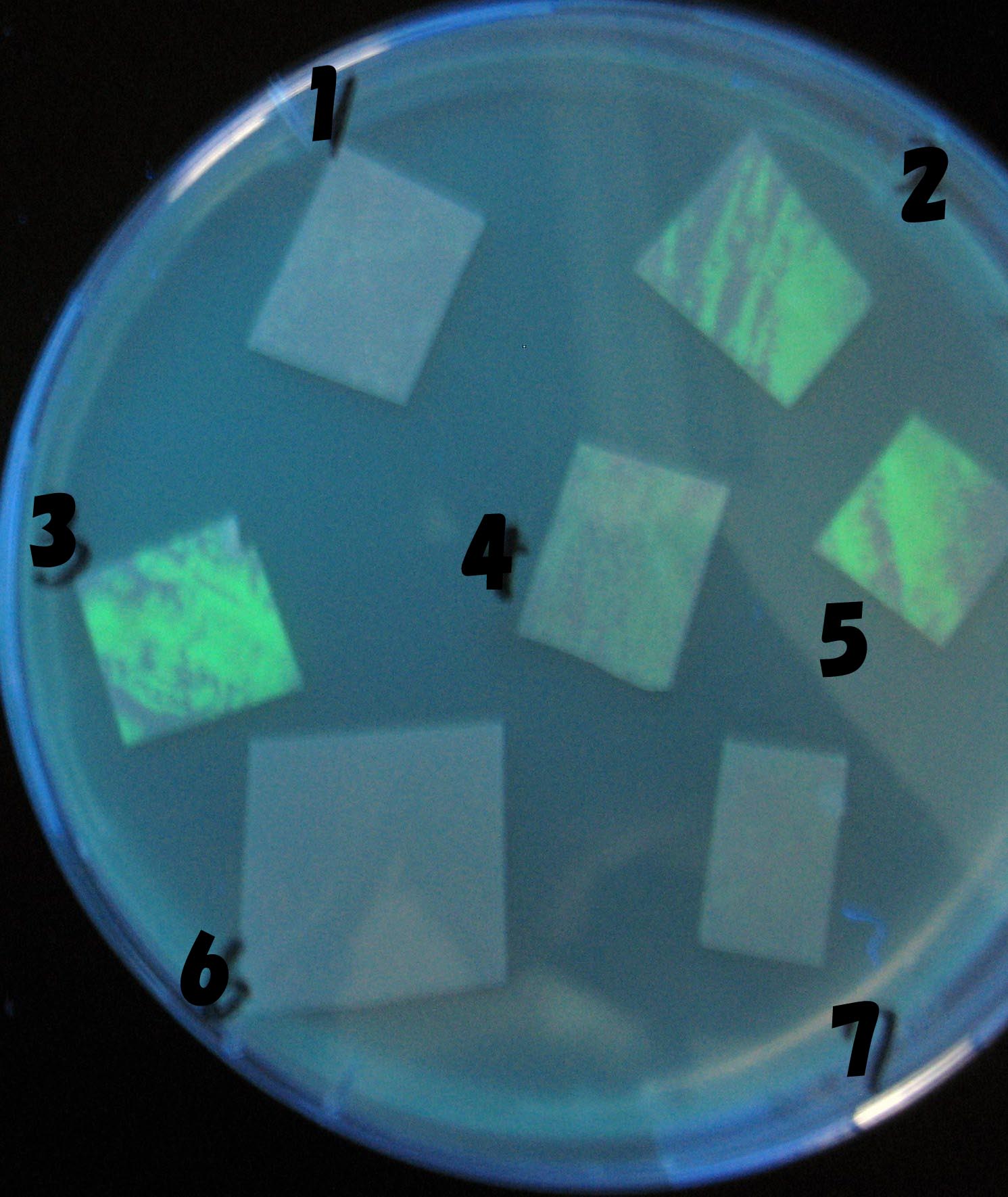
| - | + | 1000-fold dilution | |
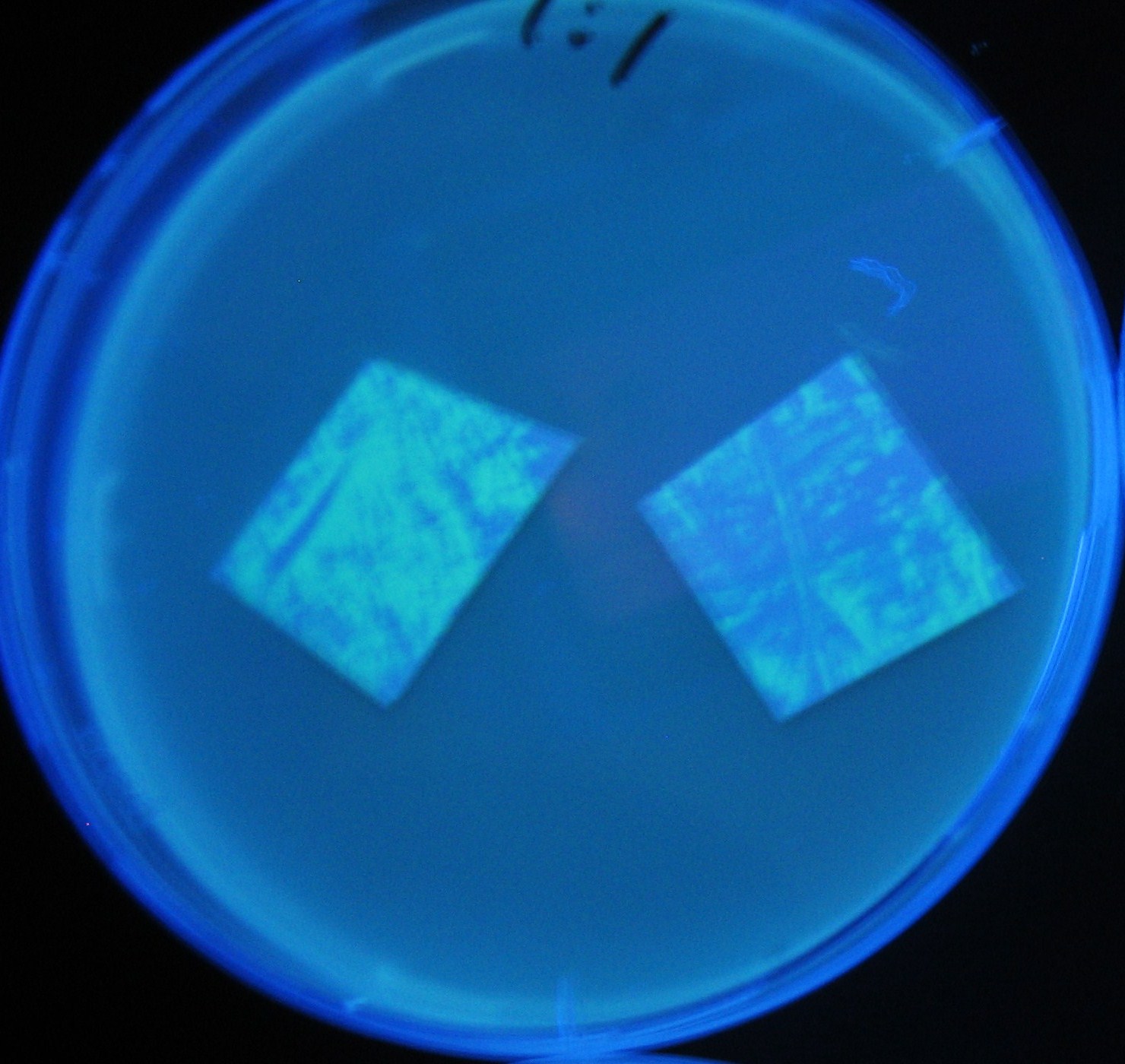
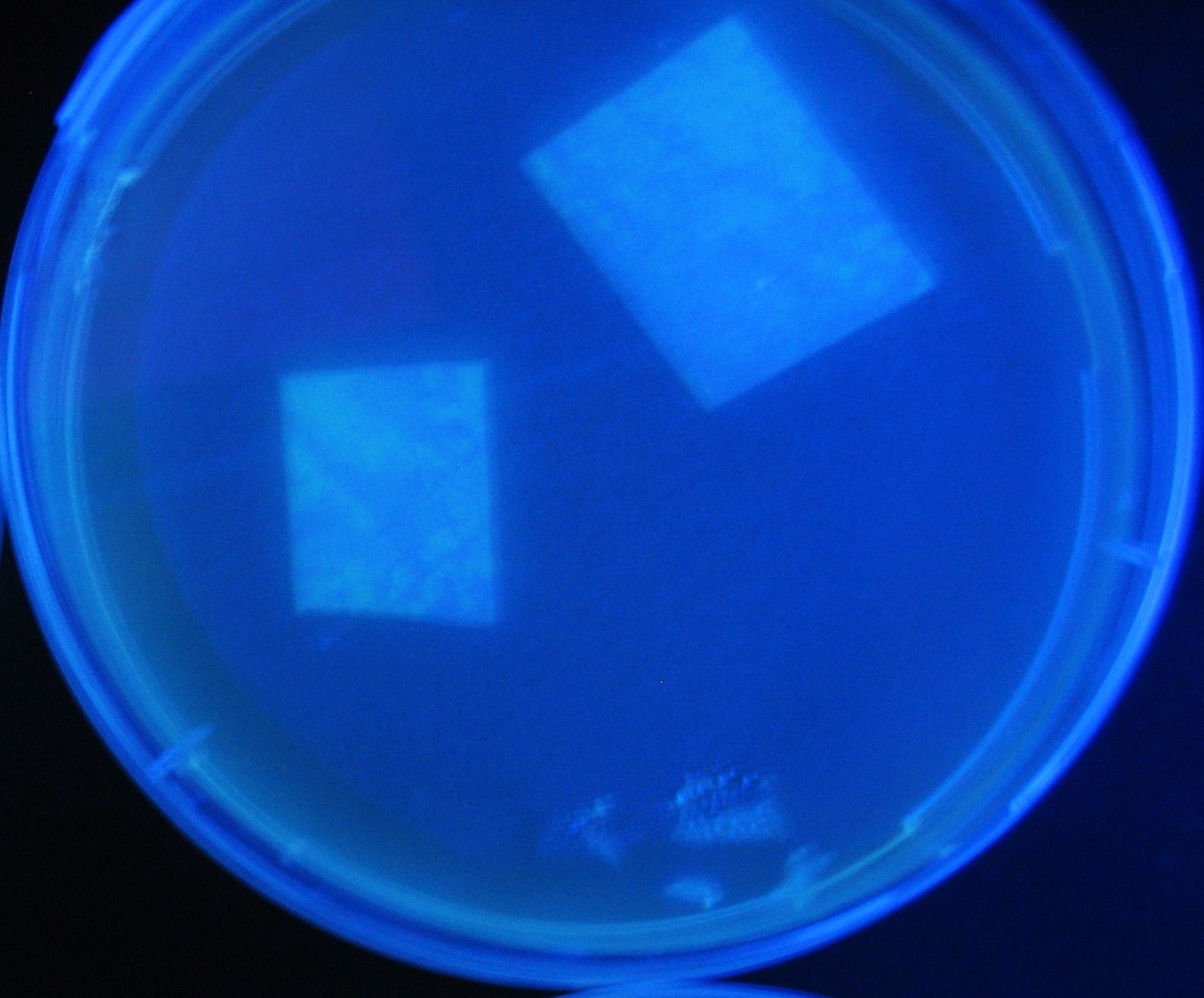
[[Image:Team-Chiba-IMG_0322-1000.JPG|160px]] | [[Image:Team-Chiba-IMG_0322-1000.JPG|160px]] | ||
[[Image:Team-Chiba-IMG_0331-1000.JPG|170px]] | [[Image:Team-Chiba-IMG_0331-1000.JPG|170px]] | ||
| Line 146: | Line 231: | ||
[[Image:Team-Chiba-IMG_0378-1000.JPG|200px]] | [[Image:Team-Chiba-IMG_0378-1000.JPG|200px]] | ||
| - | + | 0h 0.5h 1.5h | |
| + | 2.0h 2.5h | ||
| - | === | + | ===Testing different receivers-results=== |
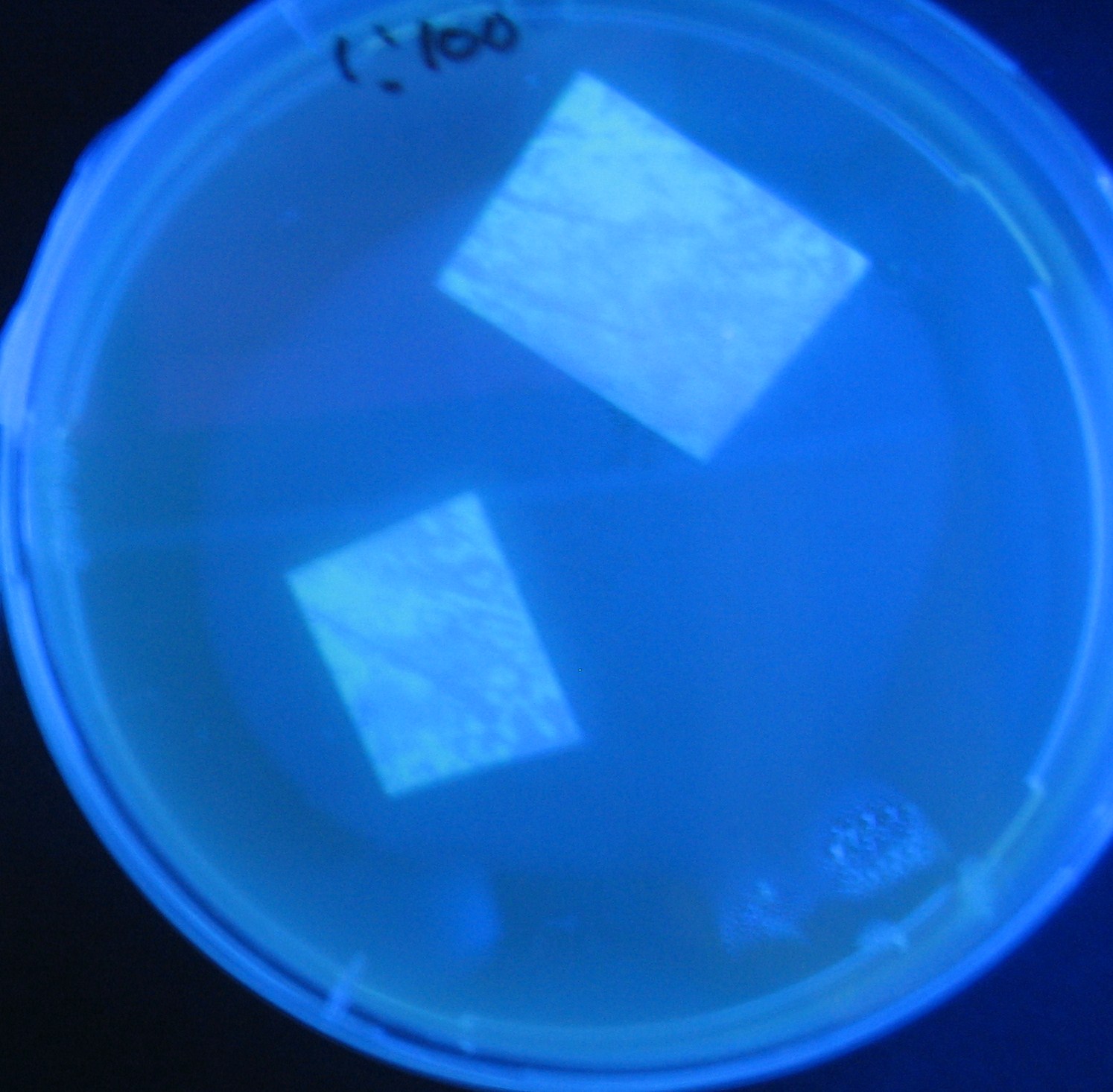
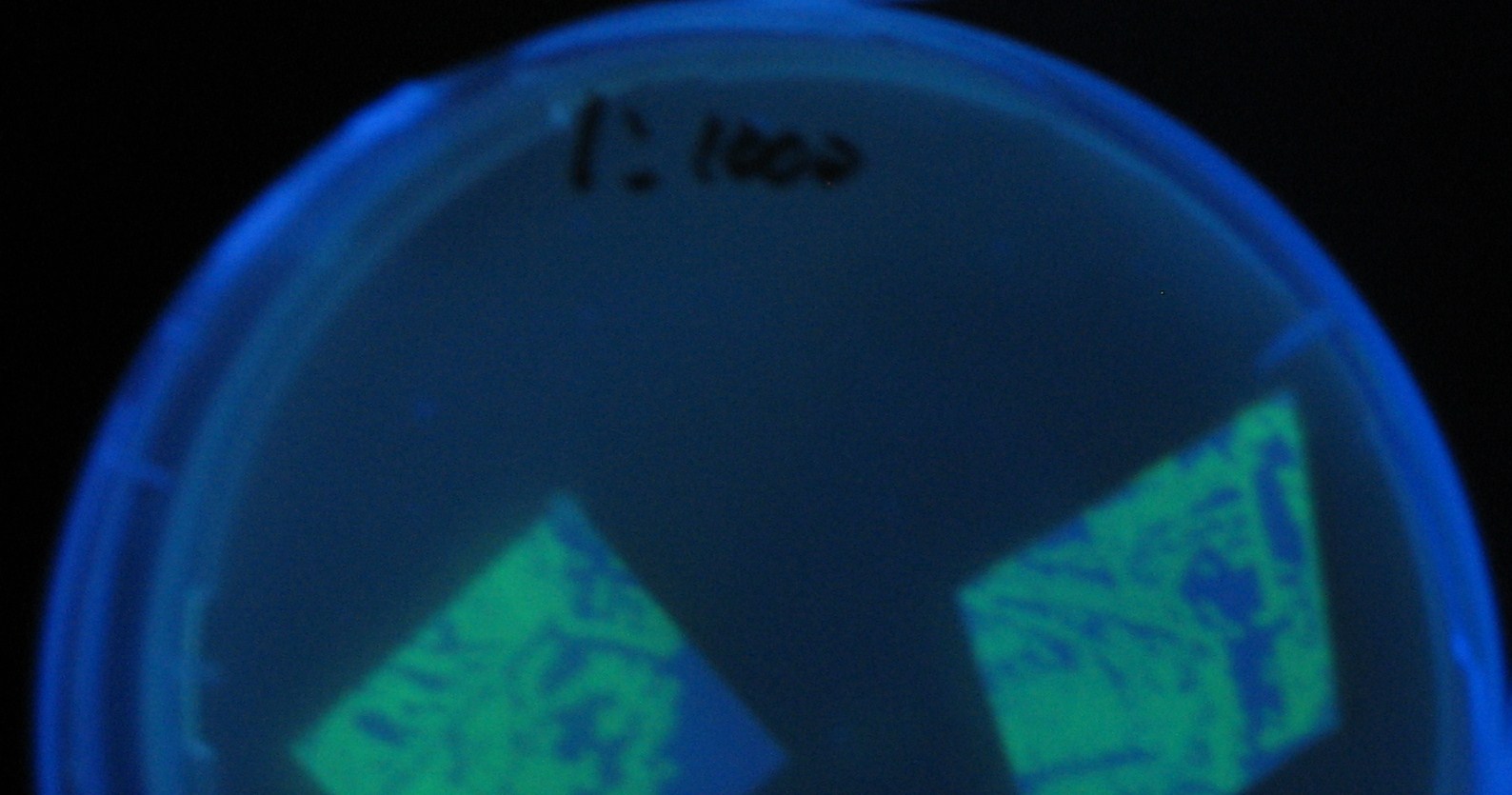
[[Image:Team-Chiba-IMG_0299.JPG.jpg|160px]] | [[Image:Team-Chiba-IMG_0299.JPG.jpg|160px]] | ||
| - | [[Image:Team-Chiba-IMG_0306-.jpg| | + | [[Image:Team-Chiba-IMG_0306-.jpg|175px]] |
| - | [[Image:Team-Chiba-IMG_0314-.jpg| | + | [[Image:Team-Chiba-IMG_0314-.jpg|160px]] |
| - | [[Image:Team-Chiba-IMG_0319-.jpg| | + | [[Image:Team-Chiba-IMG_0319-.jpg|160px]] |
| - | + | 0h 0.5h 1.0h 1.5h | |
| - | 1=N.C | + | 1=N.C |
| - | 2=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy) | + | 2=[http://partsregistry.org/Part:BBa_T9002 |
| + | BBa_T9002]:ptet-luxR-plux-GFP(high copy) | ||
| - | 3=ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032]:plux-GFP(high copy) | + | 3=ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 |
| + | BBa_J37032]:plux-GFP(high copy) | ||
| - | 4=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy) | + | 4=[http://partsregistry.org/Part:BBa_T9002 |
| + | BBa_T9002]:ptet-luxR-plux-GFP(low copy) | ||
| - | 5=ptet-mLuxR(too sensitive)-plux-GFP | + | 5=ptet-mLuxR(too sensitive)-plux-GFP |
| - | 6=N.C | + | 6=N.C |
7=ptet-luxR-plux-GFP-plac-aiiA | 7=ptet-luxR-plux-GFP-plac-aiiA | ||
| Line 173: | Line 262: | ||
'''>[[Team:Chiba/Project#Demo Experiments|Back to the project page]]''' | '''>[[Team:Chiba/Project#Demo Experiments|Back to the project page]]''' | ||
| - | {| style="color:white;background-color:Maroon" cellpadding="3" cellspacing="3" border="1" bordercolor="white" width="100%" align="center" | + | {| style="color:white;background-color:Maroon" cellpadding="3" |
| + | cellspacing="3" border="1" bordercolor="white" width="100%" | ||
| + | align="center" | ||
!align="center"|[[Team:Chiba|Home]] | !align="center"|[[Team:Chiba|Home]] | ||
!align="center"|[[Team:Chiba/Team|The Team]] | !align="center"|[[Team:Chiba/Team|The Team]] | ||
Revision as of 18:21, 29 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
Contents |
Demo Experiment ~Senders~
Method
- Pre-culture
- Picked and cultured the following glycerol stocks in 2mL of LB:
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (BW)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012
- Picked and cultured the following glycerol stocks in 2mL of LB:
BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), (XL10G)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007
BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]), (XL10G)
- Cultured at 37°C for 12h.
- Culture
- Added 6.25% each of the pre-cultures to new LB medium.
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012
- Added 6.25% each of the pre-cultures to new LB medium.
BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)])
- Cultured at 37°C for 4~5h。
- Wash
- Transfer 10mL each of the culture to 50mL centrifuge tubes.
- Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant.
- Added LB-Amp to each centrifuge tube:
- 10mL to the tube that contains
[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- 5mL to the tube that contains
[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing
[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 10mL to the tube that contains
[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing
[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 5mL to the tube that contains
[http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Mix
- Mixed the sender cells [http://partsregistry.org/Part:BBa_K084012
BBa_K084012] and [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] both with [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] at a 1:1 ratio.
- Added 100μL each to a 96-well shallow plate (as shown in the figure).
- Green part is[http://partsregistry.org/Part:BBa_K084012
- Added 100μL each to a 96-well shallow plate (as shown in the figure).
BBa_K084012]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Red part is [http://partsregistry.org/Part:BBa_K084007
BBa_K084007]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Uncolored part is [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] alone.
- Culture and observe results
Results
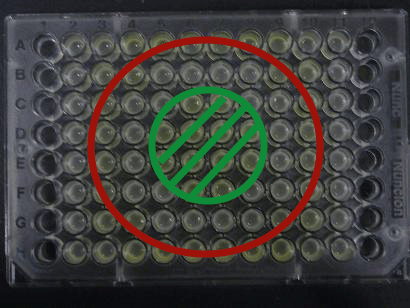 Green region: sender=LuxI,
Red circular region: sender=Las I.
Green region: sender=LuxI,
Red circular region: sender=Las I.
LuxI GFP is detected at 4h following mixing while LasI GFP is detected
after 8h, thus successfully demonstrating time-delay depending on the
sender used.
--Yoshimi 13:41, 29 October 2008 (UTC)
Demo Experiment ~Receivers~
Varying bacterial numbers: method
- Receiver(T9002) pre-incubation
- Receiver:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW)was
cultured in 2mL LB-Amp (37°C,12h)
- Pre-incubated Receiver([http://partsregistry.org/Part:BBa_T9002
BBa_T9002](BW))was plated so as to produce about 1000 colonies.
- Sender(S03623) pre-incubation
- Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) was
cultured in 50mL centrifuge tubes in 10mL of LB-Amp (37°C,12h)(2 tubes)
- Sender Wash
- Centrifuged 2 tubes containing
([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW))at 20°C, 3600rpm for 6min and discarded supernatant.
- Added 10mL LB-Amp to each tube.
- Repeated wash twice.
- Creating bacterial plates
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623
BBa_S03623](BW)) tube 1 (10mL) was mixed with LB-Amp-agar(50°C)(10ml)to produce sender containing bacterial plate-1.
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623
BBa_S03623](BW)) tube 2(100μl)was mixed with LB-Amp(9.9ml) and diluted 100-fold. 10ml of this solution was mixed with LB-Amp-agar(50°C)(10ml) and created Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate-2.
- LB-Amp pre-cultured Sender solution-2(10μl) and LB-Amp(9.99ml)
was mixed to dilute 1000-fold。10ml of this solution and LB-Amp-agar(50°C)(10ml) was mixed to create Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate-3
- Lifted with nitrocellulose
- Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW))
colony was transfered to a nitrocellulose filter and placed on each of Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate (1~3) and Sender-absent negative control plate (t=0). Determined the time required for the colonies to fluoresce depending on the bacterial concentration (100 and 1000-fold dilution).
- Method to detect fluorescence
- Plates cultured at 37°C were exposed to UV (312nm) light once
every 30 minutes to observe GFP fluorescence.
香取
Testing different receivers-methods
- Receiver&sender pre-culture
- Used Receivers were:
・[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy)
・ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032]:plux-GFP(high copy)
・[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy)
・ptet-mLuxR(too sensitive)-plux-GFP
・ptet-luxR-plux-GFP-plac-aiiA
(all BW)
Each was cultured in 2ml LB (37°C,12h) and plated so that about
1000 colonies of receiver cells will grow.
- Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) was
cultured in 10mL LB in 50mL centrifuge tubes (37°C,12h)
- sender wash
- Each receiver-containing medium was centrifuged in 50mL tubes at
de20°C, 3600rpm for 6min and supernatant discarded.
- Added 10mL LB to each tube.
- Repeated wash twice.
- Creating bacterial plates
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623
BBa_S03623](BW)) tube 1 (10mL) was mixed with LB-Amp-agar(50°C)(10ml)to produce sender containing bacterial plate-1.
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623
BBa_S03623](BW)) tube 2(100μl)was mixed with LB-Amp(9.9ml) and diluted 100-fold. 10ml of this solution was mixed with LB-Amp-agar(50°C)(10ml) and created Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate-2.
- LB-Amp pre-cultured Sender solution-2(10μl) and LB-Amp(9.99ml)
was mixed to dilute 1000-fold。10ml of this solution and LB-Amp-agar(50°C)(10ml) was mixed to create Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate-3
- Lifted with nitrocellulose
- Each Receiver colony was transfered to a nitrocellulose filter and
placed on a Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate (1~3) or a sender-absent negative control plate(t=0) to observe how receiver type affects the time taken for the colonies to display visible fluorescence.
- Method to detect fluorescence
- Plates cultured at 37°C were exposed to UV (312nm) light once
every 30 minutes to observe GFP fluorescence.
Varying bacterial numbers-results
0h 0.5h 1.5h
0h 0.5h
1.5h 2.0h
0h 0.5h 1.5h 2.0h 2.5h
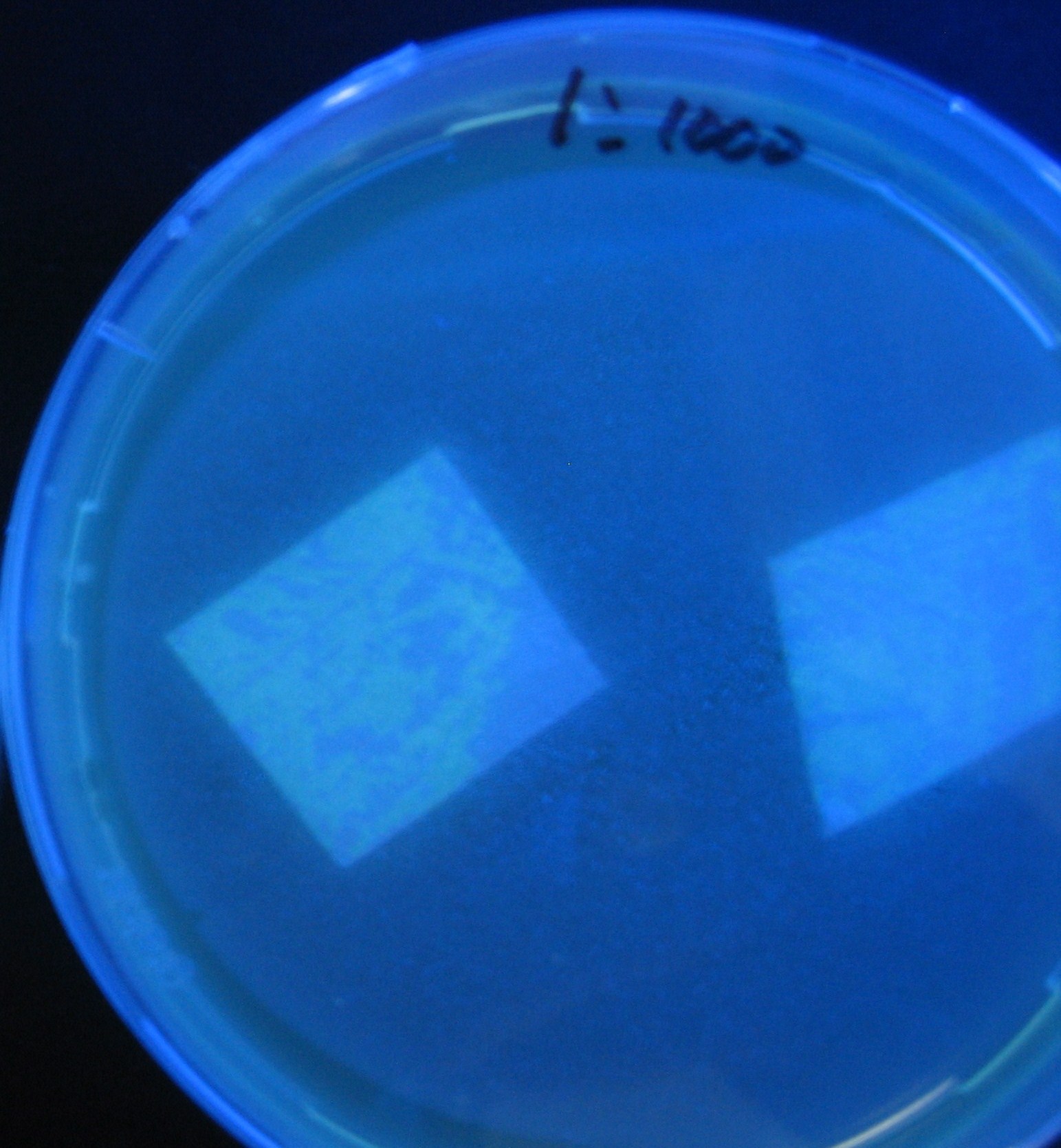
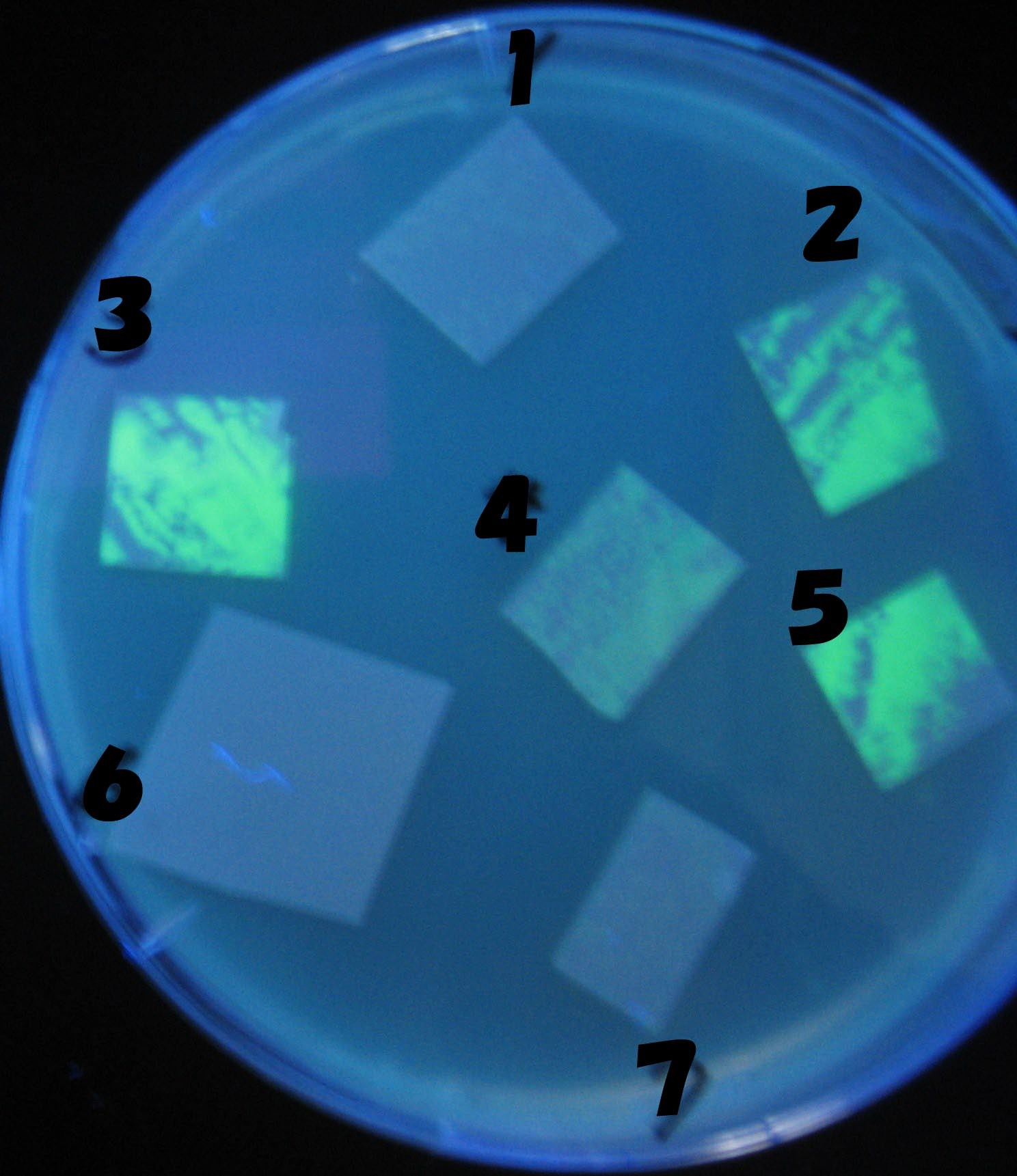
Testing different receivers-results
0h 0.5h 1.0h 1.5h
1=N.C
2=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy)
3=ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032]:plux-GFP(high copy)
4=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy)
5=ptet-mLuxR(too sensitive)-plux-GFP
6=N.C
7=ptet-luxR-plux-GFP-plac-aiiA
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"