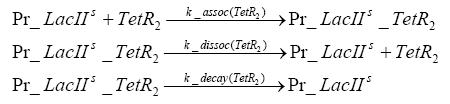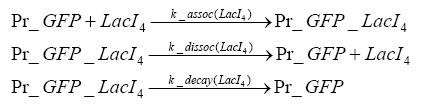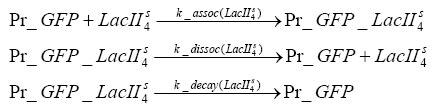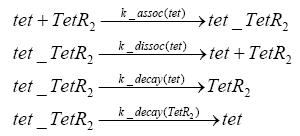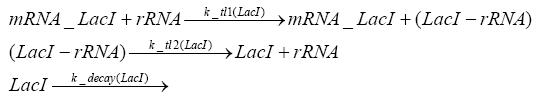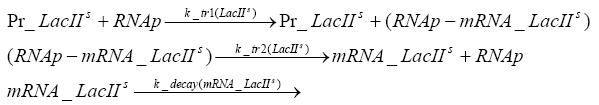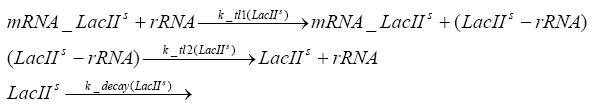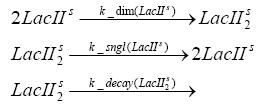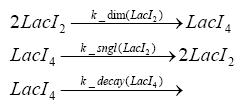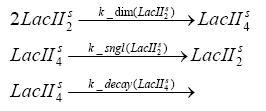Team:ETH Zurich/Modeling/Switch Circuit
From 2008.igem.org
(→Parameters) |
(→Simulation Results) |
||
| Line 36: | Line 36: | ||
=== Simulation Results === | === Simulation Results === | ||
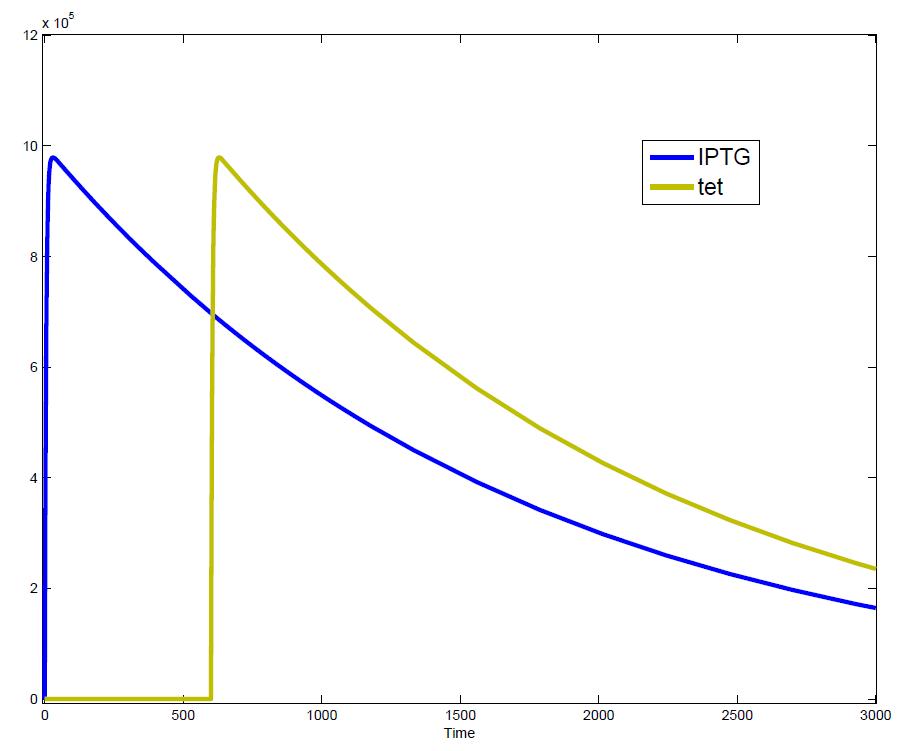
| - | [[Image:Induction2.JPG|thumb|left|420px|Input signals: Start signal (Induction with IPTG) | + | [[Image:Induction2.JPG|thumb|left|420px|Input signals: Start signal (Induction with IPTG) and Stop signal (induction with tet) after 10 min]] |
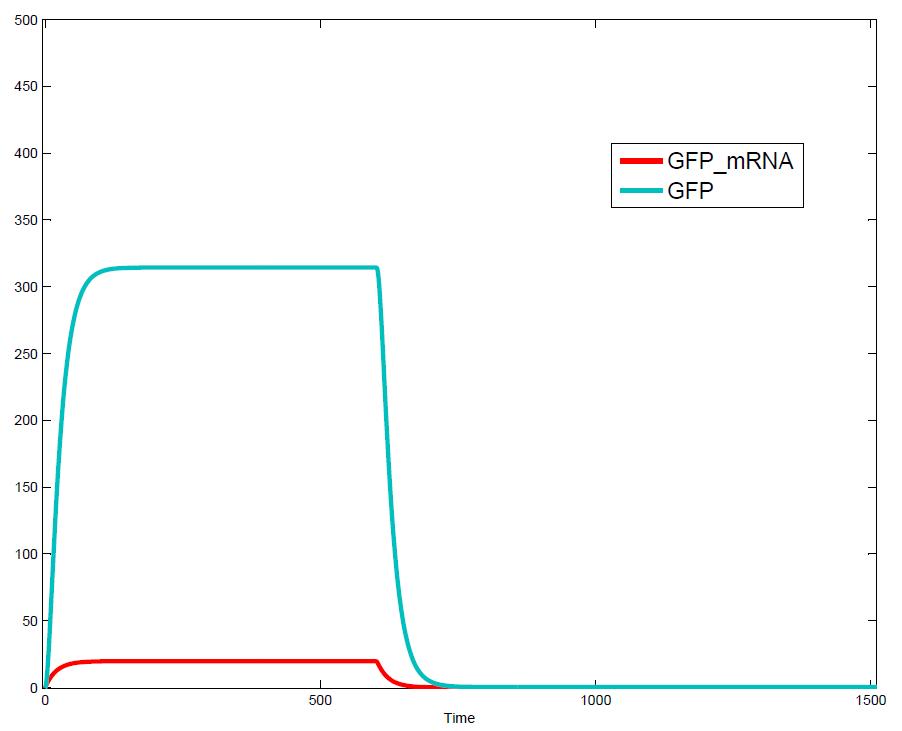
[[Image:pulse.JPG|thumb|right|428px|Output signal: expression of GFP and GFP_mRNA as a response to the input signals on the left]] | [[Image:pulse.JPG|thumb|right|428px|Output signal: expression of GFP and GFP_mRNA as a response to the input signals on the left]] | ||
<br clear="all" > | <br clear="all" > | ||
Revision as of 18:45, 29 October 2008
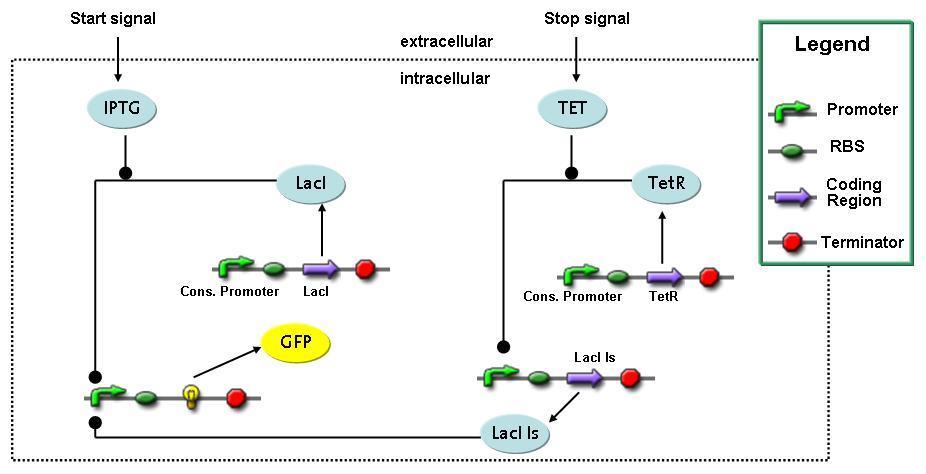
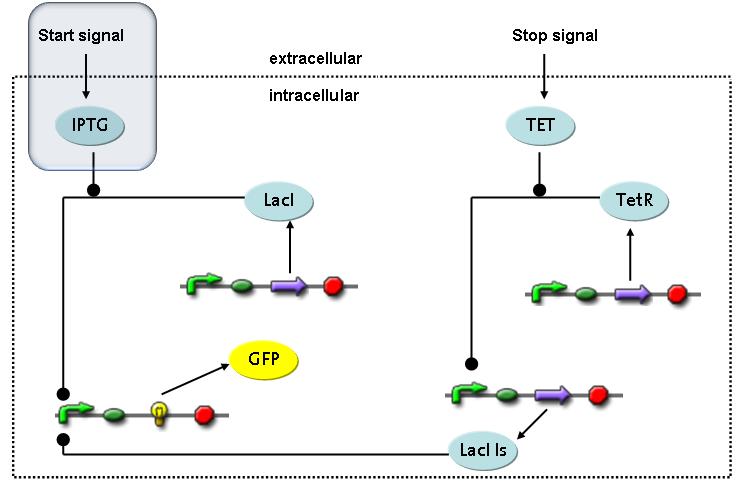
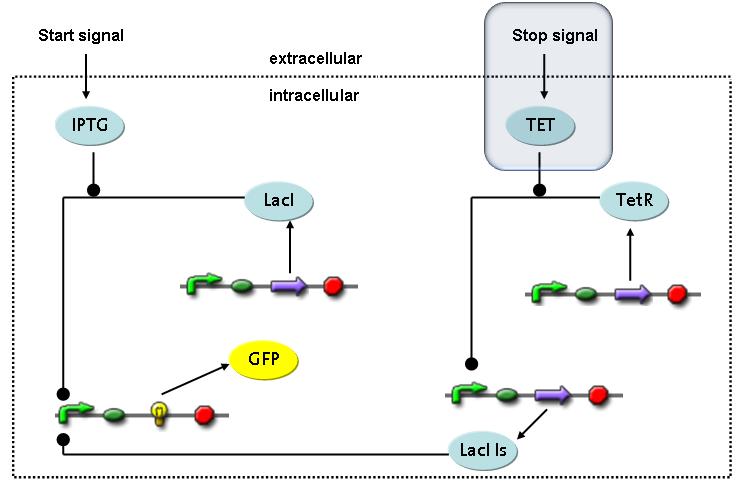
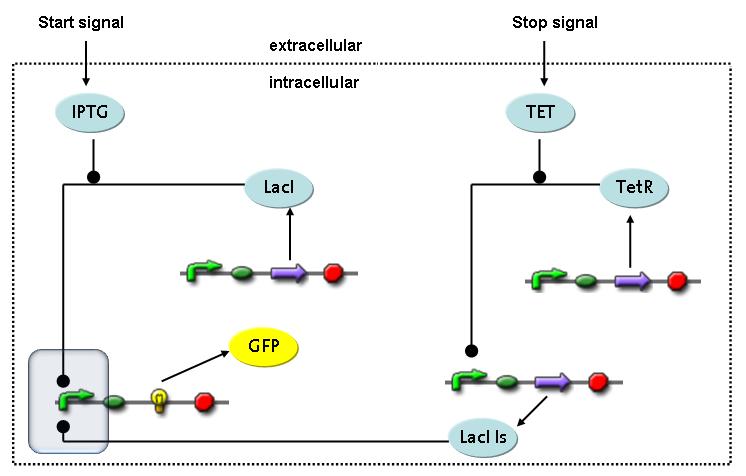
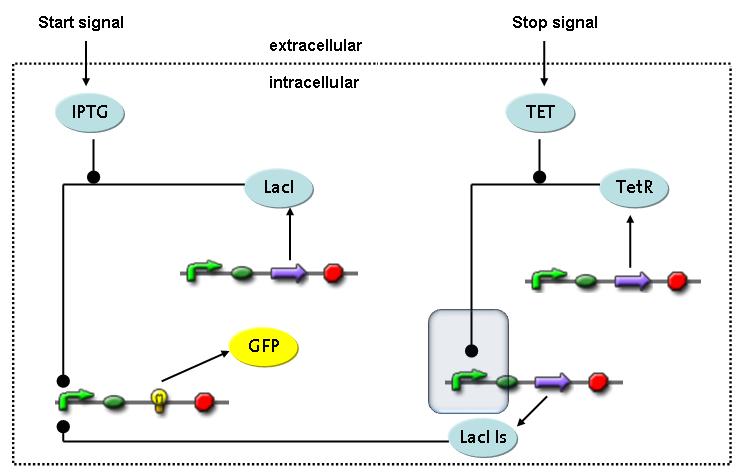
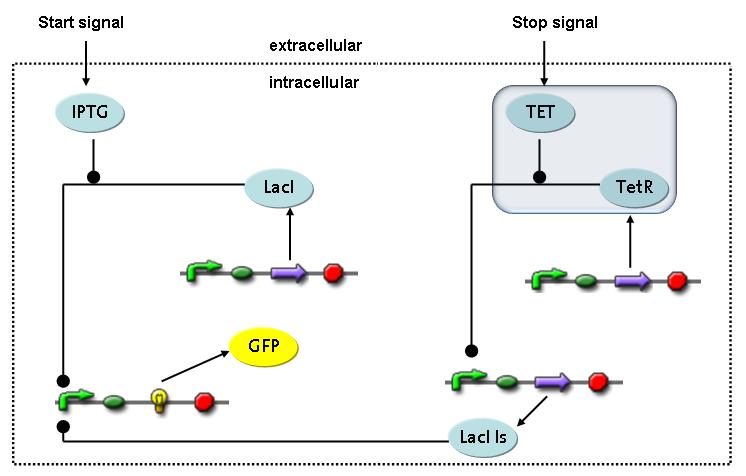
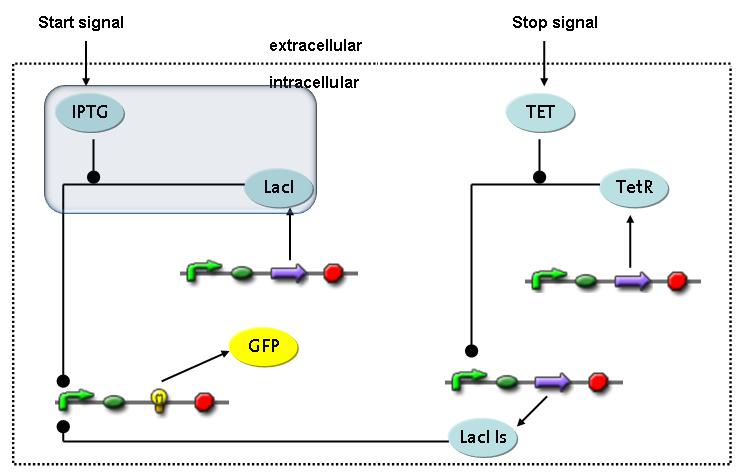
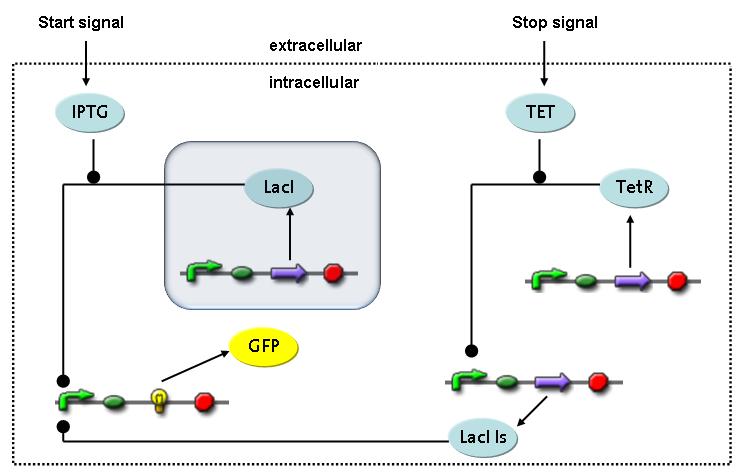
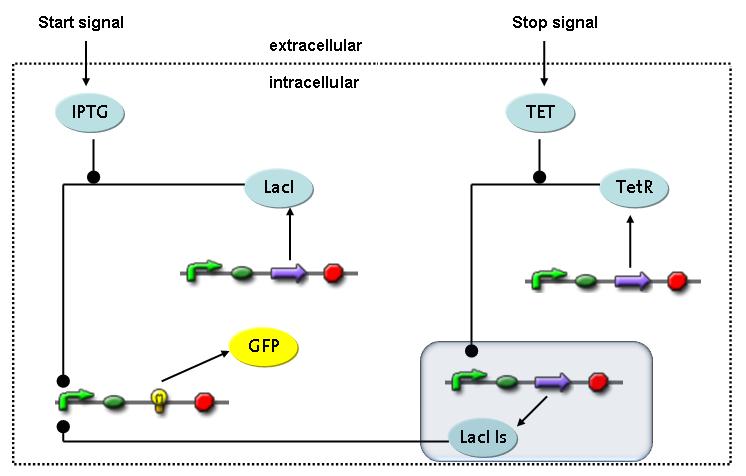
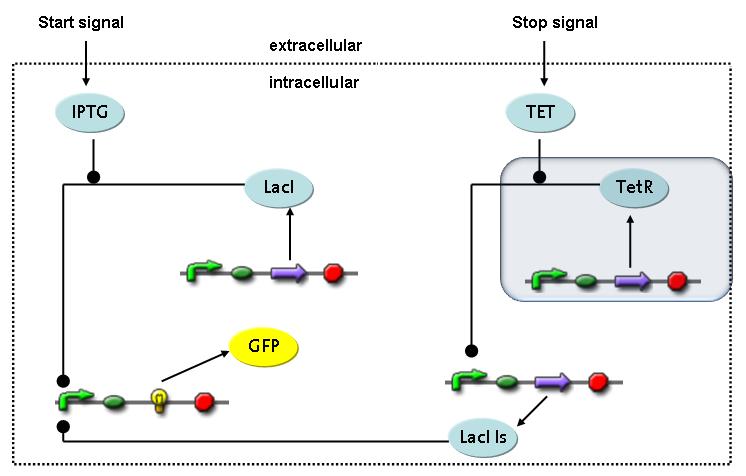
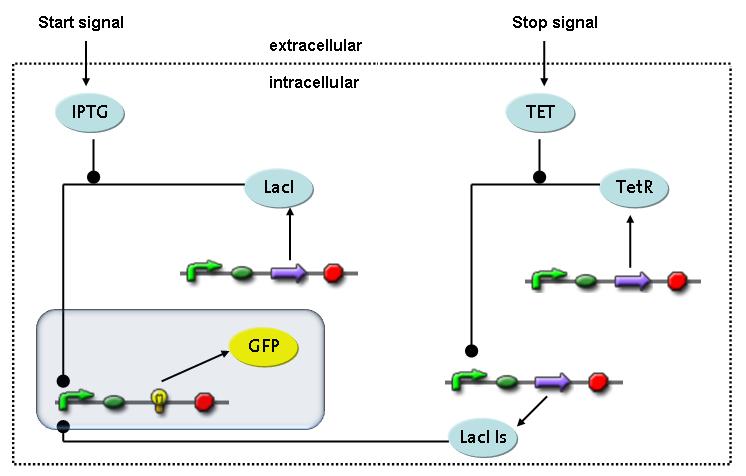
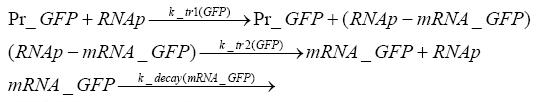
Switch CircuitWe designed a novel switching circuit consisting of two inputs and one output – a start signal initiates the synthesis of a specific protein, and a terminating signalswitches gene expression off. The goal of this system is the controlled expression of a restriction enzyme to delete genome fragments in vivo.
In order to do some preliminary experiments, the restriction enzyme has been substitued by a fluorescent protein. The basic idea of the how the system should work is as follows :
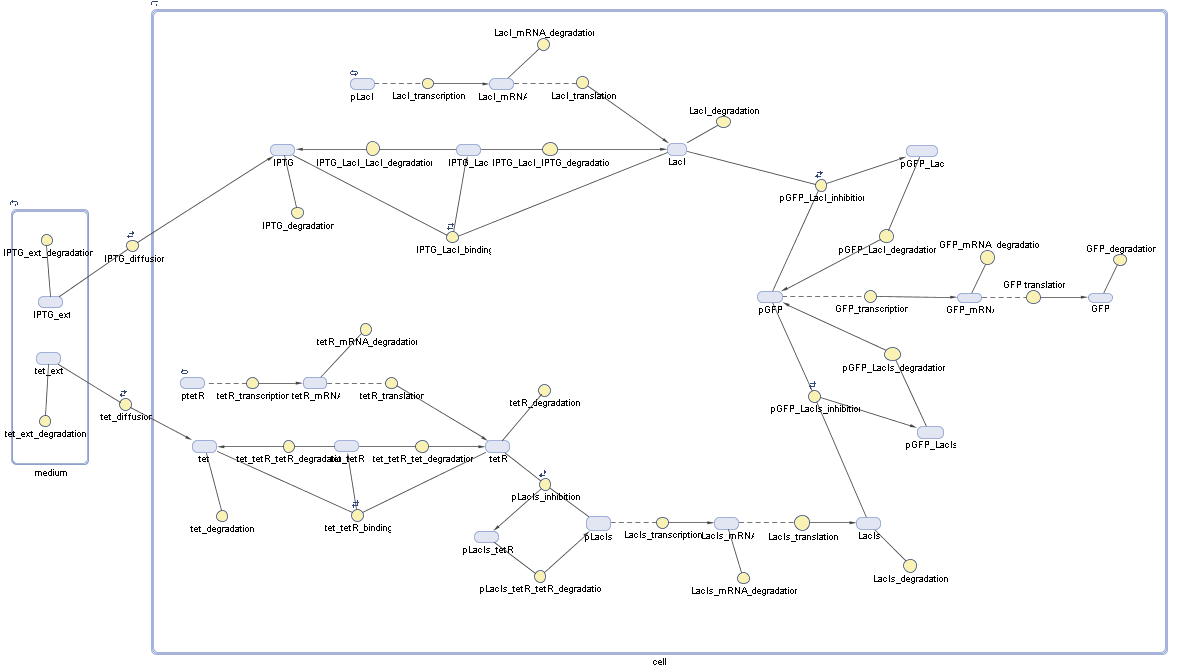
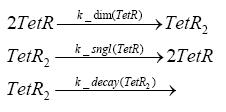
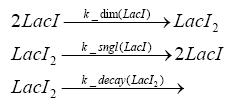
Fluorescent protein gene expression is under the control of LacI and can be induced by the addition of IPTG. In order to stop gene expression, the IPTG-sensitive LacI is replaced by IPTG-insensitive LacIS, which shuts restriction enzyme gene expression off again. The synthesis of LacIS is started by the addition of Tetracyclin to the system, which binds to the tet repressor TetR and thus de-represses the expression of the lacIs gene. The fluorescent protein is tagged, so that it is degraded faster by the Clp protease and vanishes faster from the system as its expression has been stopped. This switching circuit is described by a set of 64 chemical reactions and XX molecular species. (see "Detailed Model") In order to do a computational analysis of the circuit, this model has been simplified and implemented in MATLAB. Then the system has been simulated using ODE and stochastic solvers. Implementation and SimulationImplementationFor the implementation, different steps having no significant effects on the aspired results have been neglected for the sake of simplicity. In the transcriptions the additional step involving the RNA-Polymerase and in the translations the step involving the ribosomes have not been taken into account. Furthermore, the effects of the dimerization of TetR as well as the impacts of dimerization and tetramerization of LacI and LacIIs have not been considered in the final implementation. This simplified model still comprises more than 20 different species and over 30 kinetic reactions and has been implemented by using the Simbiology Toolbox in MATLAB. To get any useful results form the model, we performed deterministic and stochastic simulations based on the Mass-Action-Kinetics. The stochastic simulations turned out to be computationally very exhaustive but generated no further significant information compared to the deterministic simulations. Simulation Results
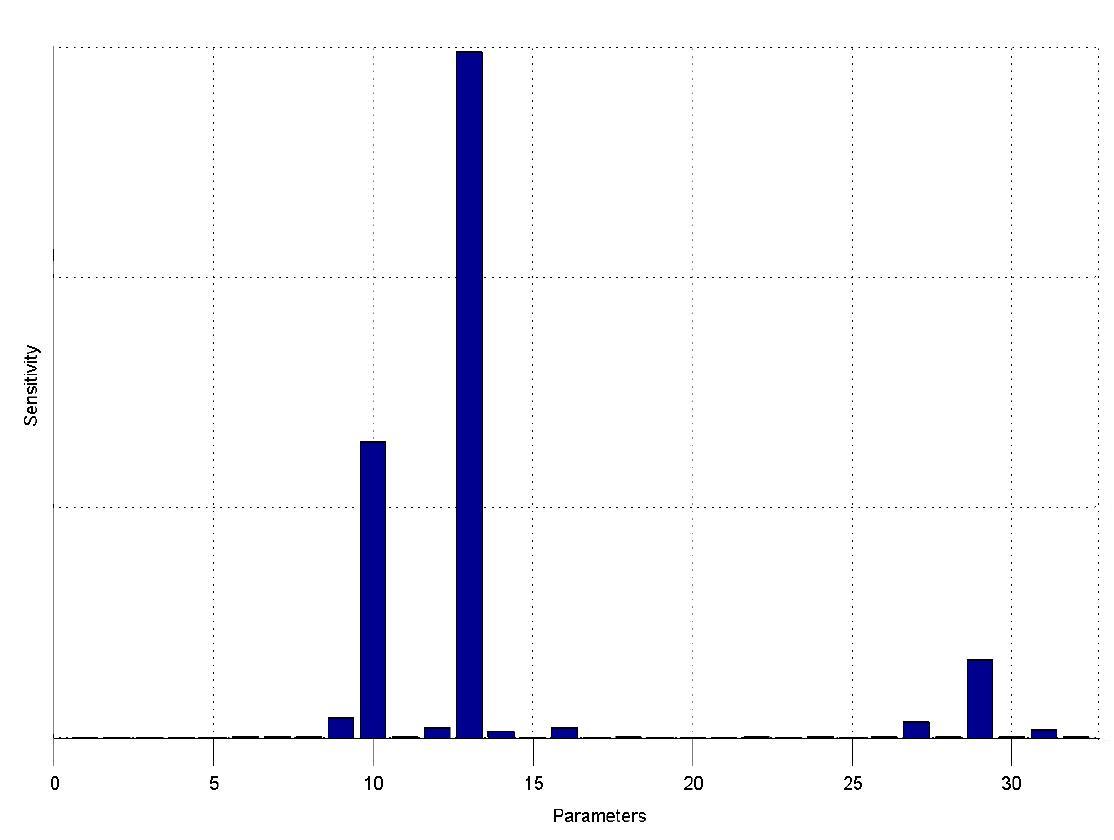
The simulations show that our system actually should create a nice pulse-shaped expression of the fluorescent protein (FP). This expression can be initiated by inducing with IPTG and stopped by subsequent addition of tet into the medium. By tagging the protein it will be degraded much faster by the Clp protease, so that the overall concentration is bounded and, after activating the stop-signal, the remaining proteins disappear quickly. Another fact that the simulations showed is that in order to get one single pulse the tet-concentration inside the medium must not reach zero before all the IPTG is degraded too. Otherwise there would still be IPTG in the system inhibiting the binding of LacI to the FP-promoter and leading to an unwanted expression of our protein of interest as the LacIIs degrades. One way to overcome this problem is by simply inducing with a much higher quantity of tet than IPTG, so that it simply takes longer for it to completely degrade or being washed away. Still another way would be to wait a bit longer after the induction with IPTG so that it has already partly vanished until switching off the FP-expression with tet. Sensitivity AnalysisWe define the sensitivity as the change of the production of the desired fluorescence protein - which is the output of our system - depending on the change of the parameters.
The sensitivity analysis shows, that the concentration of the fluorescent protein strongly depends on its decay rate (parameter 13) the decay rate of its mRNA (parameter 10) and of course the transcription and translation rates of the protein (parameters 29 and 27), which is no surprise.
We can also see that the decay rate of LacIIs (parameter 9) and the transcription rate of LacIIs (parameter 31) have an influence on the expression of the fluorescent protein.
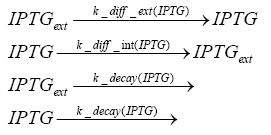
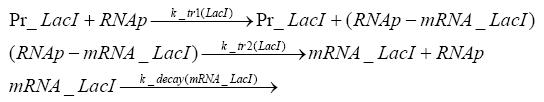
Detailed Modeldiffusion of IPTGIn order to switch on the circuit, we induce with IPTG. When IPTG is added into the medium it diffuses reversibly between the medium and the cells, where it is slowly degraded. In a chemostat extracellular IPTG is washed away. diffusion of tetThe second inducer which is used in our system is tet. This one also diffuses into the cell when it is added into the medium and degrades slowly. binding of TetR to LacIIs-promotorTetR is constitutively expressed and binds to the LacIIs promotor, inhibiting its expression. binding of LacI and LacIIs to GFP-promotorLacI which is constitutively expressed and LacIIs which is under the control of a tet repressor both can bind to the GFP promotor. binding of tet to TetRbinding of IPTG to LacItranscription and translation of LacItranscription and translation of LacIIstranscription and translation of TetRtranscription and translation of GFPdimerization of tetRdimerization and tetramerization of LacI and LacIIs
ParametersIn this section you can find all the parameters used in the simulation. Because many of the in vivo rates of the biochemical reactions we simulated are unknown, the numeric values of the kinetic parameters were mainly obtained from educated guesses (2) based on the values found in (1).
[*] degradation rates of gfp are higher because of the tagging [**] need to be that high to account for the autorepression of LacI/ in order to get a low steady state concentration of LacI of about 50 proteins (3) References(1) "Spatiotemporal control of gene expression with pulse-generating networks", Basu et al., PNAS, 2004 (2) "Genetic circuit building blocks for cellular computation, communications, and signal processing", Weiss et al., Natural Computing, 2003 (3) "Predicting stochastic gene expression dynamics in single cells", Mettetal et al., PNAS, 2006 (4) "Engineered gene circuits", Hasty et al., Nature, 2002 |
 "
"