Team:Chiba/Calendar-Home/2 September 2008
From 2008.igem.org
(Difference between revisions)
(→Team:Input) |
(→Team:Input) |
||
| Line 5: | Line 5: | ||
===Team:Input=== | ===Team:Input=== | ||
UV照射実験 | UV照射実験 | ||
| - | Incubated cultures (from Glycerol Stocks) with 2ml of LB-Ampicillin Medium for 12 hours at 37 degrees. Pre-incubated was plated so as to produce about 1000 colonies. | + | Incubated cultures (from Glycerol Stocks) with 2ml of LB-Ampicillin Medium for 12 hours at 37 degrees. Pre-incubated was plated so as to produce about 1000 colonies.(Ptet:1,Ptet-RFP:1,Prec-RFP:3) |
<BR> | <BR> | ||
| - | |||
*新たなAmpプレート8枚(2.5cm,6.5cmのそれぞれ30sec,1min,30min,1h用)に、撒いて37°C,12hたったコントロール用のPtet,Ptet-RFPのコロニーをニトロセルロースでうつしてはる。 | *新たなAmpプレート8枚(2.5cm,6.5cmのそれぞれ30sec,1min,30min,1h用)に、撒いて37°C,12hたったコントロール用のPtet,Ptet-RFPのコロニーをニトロセルロースでうつしてはる。 | ||
*PrecA-RFPのプレートにUVを照射する。UVからの距離は2.5cmと,6.5cmの2パターンで実験する。Prec-RFPのコントロール用は暗所に置いておく。 | *PrecA-RFPのプレートにUVを照射する。UVからの距離は2.5cmと,6.5cmの2パターンで実験する。Prec-RFPのコントロール用は暗所に置いておく。 | ||
Revision as of 23:06, 29 October 2008
1 September 2008 <|> 3 September 2008
Contents |
Laboratory work
Team:Input
UV照射実験
Incubated cultures (from Glycerol Stocks) with 2ml of LB-Ampicillin Medium for 12 hours at 37 degrees. Pre-incubated was plated so as to produce about 1000 colonies.(Ptet:1,Ptet-RFP:1,Prec-RFP:3)
- 新たなAmpプレート8枚(2.5cm,6.5cmのそれぞれ30sec,1min,30min,1h用)に、撒いて37°C,12hたったコントロール用のPtet,Ptet-RFPのコロニーをニトロセルロースでうつしてはる。
- PrecA-RFPのプレートにUVを照射する。UVからの距離は2.5cmと,6.5cmの2パターンで実験する。Prec-RFPのコントロール用は暗所に置いておく。
- UV照射後30sec,1min,30min,1h経過したらそれぞれ(2.5cmでUV当てたもの、6.5cmでUV当てたもの、コントロール用)のプレートからニトロセルロースでコロニーをうつしとり、あらかじめコントロールをはっておいたAmpプレートにはりつけた。
- それぞれのプレートでUVが照射されてからどのくらいの時間でRFPが発現するのかを調べるためにUV照射してから、0min,10min,30min,1h,2h,3h,4h,5h,6h後にスキャナーで取り込んで色の変化を見る。
- 結果
- PrecA-RFPにUVを当てたものはいづれもRFPが目で確認できなかった。
- Incubated cultures(from Glycerol Stocks) with 2mL of LB-Ampicillin Medium for 12 hours at 37 degrees.
OD:#UV⊕:5.21,UV⊖(negative control):5.38
- moved the cultures to small plates and started UV-irradiation.(wavelength:254nm,distance from the UV lamp to the cultures were 7.5cm.Put the negartive control in adark place.
- covered the plates with polyethylene wrap.
- after irradiation(Sample:0min,10min,30min,1h,2h,4h,6h,8h)(Negative Control:0h,1h,4h,8h),agitate the culture and
よく攪拌してから、20μl採取。
- diluted each cultures with LB-ampicillin medium 104-fold and 105-fold (volume/volume).
- incoculated 20μl of the cultures and incubated for 12 hours at 37 degrees.
- counted the CFU(determined viable cell count).
Team:Communication
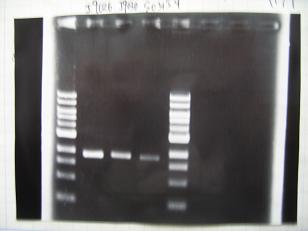
- (31/8)--->Gel Check
- (1/9)---> Colony PCR
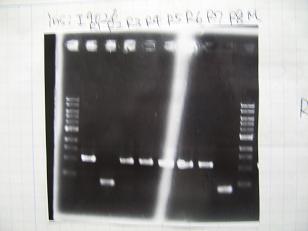
- Colony PCR of 8 colonies from ligation plates (1/9:(1)[http://partsregistry.org/Part:BBa_K084009 BBa_K084009](R1~R8),(2)[http://partsregistry.org/Part:BBa_K084010 BBa_K084010](C1~C8)) and one from control plate([http://partsregistry.org/Part:BBa_F2620 BBa_F2620](2007)).
DNA Template 1 dNTP mix 5 Foward Primer 0.3 Reverse Primer 0.3 DNA polymerase TAQ 0.5 Thermopol Buffer 3 dH2O 20.5 TOTAL 30μL
- 95°C,5min -> ( 95°C,1min -> 52°C,1min -> 72°C,1min )・・・25cycles -> 72°C,10min -> 6°C
--->Gel CheckSample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6μl - From left;
- Plac+RBS+RhlI+LVA
- R1 -> OK
- R2 -> Bad
- R3~R7 -> OK
- R8 -> Bad
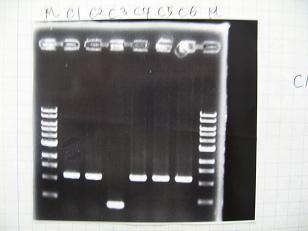
- From left;
- From left;
- Plac+RBS+CinI+LVA
- C1,C2 -> OK
- C3 -> Bad
- C4~C6 -> OK
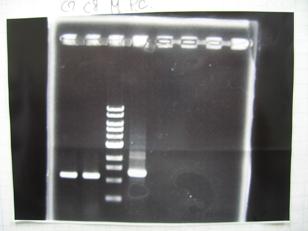
- From left;
- Plac+RBS+CinI+LVA
- C7,C8 -> OK
- [http://partsregistry.org/Part:BBa_F2620 BBa_F2620](2007):Positive control -> OK
- --->(3/9)Mini prep
(1/9)--->Liquid Culture- Cultured the following cells (2mL LB-Amp, at 37°C, 7 hours)
- from transformed plates:
- [http://partsregistry.org/Part:BBa_K084007 BBa_K084007](Plac+RBS+LasI, Competent Cells : JW1908)
- [http://partsregistry.org/Part:BBa_K084008 BBa_K084008](Plac+RBS+RhlI, Competent Cells : JW1908)
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002](Ptet+RBS+LuxR+GFP, Competent Cells : JW1908)
- from Glycerol Stock:
- [http://partsregistry.org/Part:BBa_S03623 BBa_S03623](Ptet+RBS+LuxI, Competent Cells : JW1908)
- from transformed plates:
--->(3/9)Phenotype test
- Competent cells : XL10G 30μL
- [http://partsregistry.org/Part:BBa_C0161 BBa_C0161](2007)
- [http://partsregistry.org/Part:BBa_C0161 BBa_C0161](2006)
- [http://partsregistry.org/Part:BBa_C0261 BBa_C0261](2007)
- [http://partsregistry.org/Part:BBa_C0261 BBa_C0261](2006)
--->(4/9)Mini prep
Team:Output
Colony PCR
- [http://partsregistry.org/Part:BBa_R0010 BBa_R0010]+[http://partsregistry.org/Part:BBa_J52008 BBa_J52008]
Sample No. 1 culture 1 Fwd primer 1.5 Rev primer 1.5 Thermo pol Buffer 3 dNTP mix 3 Taq DNA pol (NEB) 0.2(1 unit) dH2O 19.8 TOTAL 30μl
-->95°C 5 min -->(95°C 1min -->50°C 30sec -->72°C 1min)x25 -->72°C 10min- [http://partsregistry.org/Part:BBa_R0079 BBa_R0079]
- [http://partsregistry.org/Part:BBa_R0071 BBa_R0071]
- [http://partsregistry.org/Part:BBa_R0077 BBa_R0077]
- [http://partsregistry.org/Part:BBa_R0078 BBa_R0078]
- [http://partsregistry.org/Part:BBa_R0062 BBa_R0062]
 "
"