Team:Michigan/Project/Fabrication
From 2008.igem.org
(Difference between revisions)
| Line 54: | Line 54: | ||
!width="70%" align="justify" valign="top" style="background:transparent; color:navy"| | !width="70%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| - | + | <br><font size=3 color=royalblue>Constitutive Promoter</font> | |
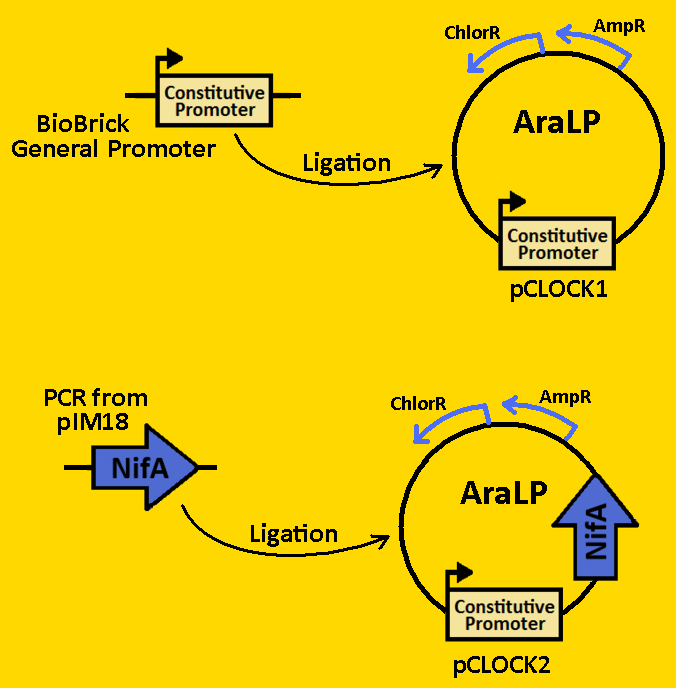
| + | *pArabLP was digested with EcoRI and NdeI | ||
| + | *BioBrick constitutive promoters were double digested EcoRI and NdeI. | ||
| + | *Digests were purified using QIAGEN Purification Kit | ||
| + | *Ligation was done overnight in 4⁰C. | ||
| + | *pArabLP-CP was then transformed into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were isolated by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then digested with EcoRI and SpeI to ensure proper ligation overnight in 37⁰C. | ||
| + | *Analytical gel was then run to check fragment size of approximately 50bp. | ||
| + | |||
| + | <br><font size=3 color=royalblue>NifA</font> | ||
| + | *NifA was amplified from pRT22 using primers that had EcoRI, XbaI, NdeI and SpeI, PstI restriction sites flanking the 1560bp mutant sequence. | ||
| + | *The NifA sequence was then purified using the QIAGEN QIAquick PCR Purification Kit. | ||
| + | *NifA was double digested with NdeI and SpeI and left overnight in 37⁰C. | ||
| + | *pArabLP-CP was double digested with NdeI and SpeI also overnight at 37⁰C. | ||
| + | *Digests were purified using QIAGEN Purification Kit | ||
| + | *Ligation was done overnight in 4⁰C. | ||
| + | *pArabLP-NifA was then transformed into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were isolated by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then digested with EcoRI and SpeI to ensure proper ligation overnight in 37⁰C. | ||
| + | *Analytical gel was then run to check fragment size of approximately 1650bp. | ||
| + | |||
!width="10%" align="justify" valign="top" style="background:transparent; color:navy"| | !width="10%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| Line 67: | Line 90: | ||
!width="70%" align="justify" valign="top" style="background:transparent; color:navy"| | !width="70%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| - | + | <br><font size=3 color=royalblue>NifHp</font> | |
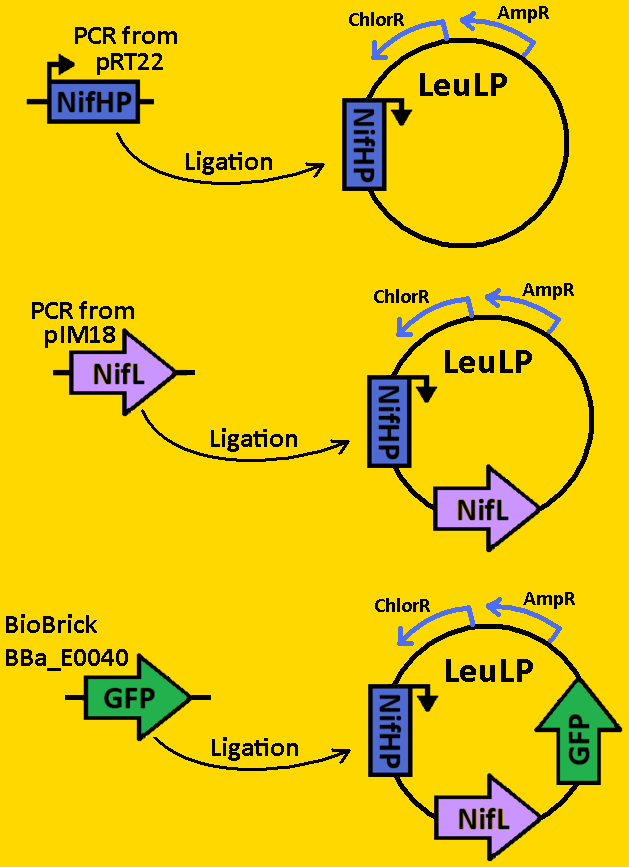
| + | *The NifH promoter (NifHp) was amplified from pRT22 using primers that had EcoRI, XbaI and NdeI, SpeI, PstI restriction sites flanking the 290bp sequence. | ||
| + | *The NifHp sequence was then purified using the QIAGEN QIAquick PCR Purification Kit. | ||
| + | *NifHp was double digested with EcoRI and SpeI and left overnight in 37⁰C. | ||
| + | *pLeuLP was double digested with EcoRI and SpeI also overnight at 37⁰C. | ||
| + | *Digests were purified using QIAGEN Purification Kit | ||
| + | *Ligation was done overnight in 4⁰C. Newly formed plasmid is renamed to pCLOCK1 | ||
| + | *pCLOCK1 was then transformed into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were isolated by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then digested with EcoRI and SpeI to ensure proper ligation overnight in 37⁰C. | ||
| + | *Analytical gel was then run to check fragment size of approximately 300bp. | ||
| + | |||
| + | <br><font size=3 color=royalblue>NifL</font> | ||
| + | *NifL was amplified from pIM18 using primers that had EcoRI, XbaI, NdeI and SpeI, PstI restriction sites flanking the 1590bp mutant sequence. | ||
| + | *The NifL sequence was then purified using the QIAGEN QIAquick PCR Purification Kit. | ||
| + | *NifL was double digested with NdeI and SpeI and left overnight in 37⁰C. | ||
| + | *pClock1 was double digested with NdeI and SpeI also overnight at 37⁰C. | ||
| + | *Digests were purified using QIAGEN Purification Kit | ||
| + | *Ligation was done overnight in 4⁰C. Newly formed plasmid is renamed to pCLOCK2 | ||
| + | *pCLOCK2 was then transformed into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were isolated by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then digested with EcoRI and SpeI to ensure proper ligation overnight in 37⁰C. | ||
| + | *Analytical gel was then run to check fragment size of approximately 2000bp. | ||
| + | |||
| + | <br><font size=3 color=royalblue>GFP </font> | ||
| + | *pClock2 was digested with EcoRI and SpeI | ||
| + | *BioBrick BBa_E0040 that contained GFP was digested with EcoRI and XbaI in sequential digests. | ||
| + | *Digests were purified using QIAGEN Purification Kit | ||
| + | *Ligation was done overnight in 4⁰C. | ||
| + | *pCLOCK2 was then transformed into E.coli competent cells and grown overnight on LB+Amp plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp overnight in 37⁰C. | ||
| + | *Plasmids from cells were isolated by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then digested with EcoRI and SpeI to ensure proper ligation overnight in 37⁰C. | ||
| + | *Analytical gel was then run to check fragment size of approximately 2700bp. | ||
!width="10%" align="justify" valign="top" style="background:transparent; color:navy"| | !width="10%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
Revision as of 02:23, 30 October 2008
|
|---|
|
Project FabricationWe will be using landing pads to insert the Sequestillator onto the chromosome of E. coli. Noisy behavior has proven detrimental to clock studies throughout the past, and we hope to reduce the noise in our system using landing pads.
Landing Pad Plans for Sequestillator
Specific Fabrication TechniquesActivator Operon
Repressor Operon
|
|---|
 "
"