Team:Chiba/Project/Experiments:Signal Molecule Quencher
From 2008.igem.org
m |
(→Experiments) |
||
| Line 33: | Line 33: | ||
===Experiments=== | ===Experiments=== | ||
| - | + | Experiments; | |
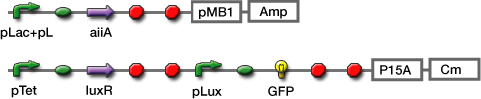
| - | - | + | Co-transformation; We constructed the circuit below. AiiA is placed on the high-copy plasmid under the control of Lac promoter. It was co-transformed with the LuxR/ Plux reporter on the p15A plasmid. |
| - | + | Communication; The resultant "AiiA/LuxR reporter" was co-cultured with LuxI sender and the fluorescence was monitored over time. As a control, we conducted the same experiment with LuxR reporter without AiiA plasmid. | |
| - | + | ||
| - | + | ||
| - | + | Notes | |
| - | + | Sender; LuxI plasmid transformed into E.coli strains (JW1908) | |
| + | Receiver; LuxR-gfp plasmid transformed into E.coli strains (JW1908) | ||
| + | Culture/ Cndn. | ||
| + | |||
| + | 1. Both Sender and Receiver (+/- AiiA) were inoculated into small (2mL) culture and was shaken separately for 12h (at 37C) | ||
| + | 2. Inoculated into flesh media, shaken until cell density hit 2.0 in OD600 | ||
| + | 3. Washed the cell and re-suspended. Cell density checked. | ||
| + | 4. Mixed Sender and Receiver (Sender/Receiver 1000μl/1000μl). | ||
| + | 5. Incubated at 30°C. | ||
| + | 6. Time-chased the fluorescence (485nm(excitation) and 527nm(emission)) by gfp. | ||
| - | |||
{| class="tbl" | | {| class="tbl" | | ||
|- | |- | ||
Revision as of 01:17, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
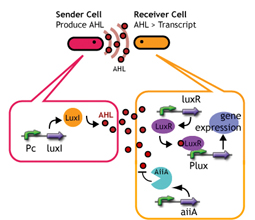
AiiA Receiver
Design
Design
|
AiiA added to Lux reporter
|
--Masahiro 00:40, 30 October 2008 (UTC)
Experiments
Experiments; Co-transformation; We constructed the circuit below. AiiA is placed on the high-copy plasmid under the control of Lac promoter. It was co-transformed with the LuxR/ Plux reporter on the p15A plasmid.
Communication; The resultant "AiiA/LuxR reporter" was co-cultured with LuxI sender and the fluorescence was monitored over time. As a control, we conducted the same experiment with LuxR reporter without AiiA plasmid.
Notes
Sender; LuxI plasmid transformed into E.coli strains (JW1908) Receiver; LuxR-gfp plasmid transformed into E.coli strains (JW1908) Culture/ Cndn.
1. Both Sender and Receiver (+/- AiiA) were inoculated into small (2mL) culture and was shaken separately for 12h (at 37C) 2. Inoculated into flesh media, shaken until cell density hit 2.0 in OD600 3. Washed the cell and re-suspended. Cell density checked. 4. Mixed Sender and Receiver (Sender/Receiver 1000μl/1000μl). 5. Incubated at 30°C. 6. Time-chased the fluorescence (485nm(excitation) and 527nm(emission)) by gfp.
|
|
| [http://partsregistry.org/Part:BBa_S03623 BBa_S03623 (AHL sender)] |
|
|
Method
- Transformed Sender into E.coli strains(JW1908) and Receivers into E.coli strain(JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C 12h.
- Inoculated again at 37°C upto about OD600=2.0
- Washed them.
- Mixed them (Sender:Receiver=1000μl:1000μl).
- Incubated at 30°C.
- Measured intensity of green fluorescence at regular time intervals.
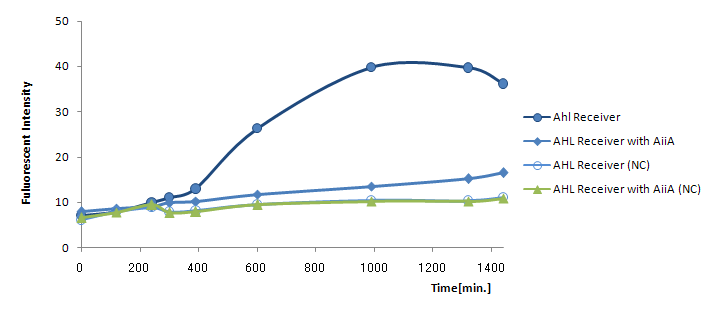
Result & Discussion
24時間後の蛍光強度を比較すると最大強度は1/4に下がっている。AiiAを発現させると、GFPの発現自体が大幅に減ってしまったので、AHL自体を減らしてしまうと発現量の最大値が小さくなってしまうことがわかった。つまり今回の実験ではAiiAがAHLを分解しすぎてしまっている言える。 よって本実験ではAiiA generatorがハイコピープラスミドに乗っていたので、AiiA generatorをローコピープラスミドに乗せ換えてTime Delay Testを行いたい。
transfer curveがシグモイドではなく、強度は低いが少なくともAHL Reporterは時間に比例して発現していた。 よって[http://partsregistry.org/Part:BBa_R0063 BBa_R0063 (Medium strength promoter in the absence of LuxR/HSL)]でAiiAを合成すれば、AHLが低濃度のときだけAiiAを合成し、いったんAHLがある一定の濃度を超えてしまえばAiiAによるAHL分解量は下降の一途を辿る。この遺伝子回路を用いれば新たなタイムディレイ作成の方法が提案できると考える。
References
- [http://www.jbc.org/cgi/content/full/279/14/13645 Wang et al.:Specificity and Enzyme Kinetics of the Quorum-quenching N-Acyl Homoserine Lactone Lactonase (AHL-lactonase).J. Biol. Chem.279(14),13645-13651,2004.]
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"