Team:Chiba/Project/Experiments:Signal Molecule Quencher
From 2008.igem.org
(→Experiments) |
(→Experiments) |
||
| Line 44: | Line 44: | ||
Culture/ Cndn. | Culture/ Cndn. | ||
| - | + | #Both Sender and Receiver (+/- AiiA) were inoculated into small (2mL) culture and was shaken separately for 12h (at 37°C) | |
| - | + | #Inoculated into flesh media, shaken until cell density hit 2.0 in OD600 | |
| - | + | #Washed the cell and re-suspended. Cell density checked. | |
| - | + | #Mixed Sender and Receiver (Sender/Receiver 1000μl/1000μl). | |
| - | + | #Incubated at 30°C. | |
| - | + | #Time-chased the fluorescence (485nm(excitation) and 527nm(emission)) by gfp. | |
{| class="tbl" | | {| class="tbl" | | ||
Revision as of 01:26, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
AiiA Receiver
Design
Design
|
AiiA added to Lux reporter
|
--Masahiro 00:40, 30 October 2008 (UTC)
Experiments
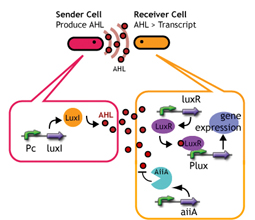
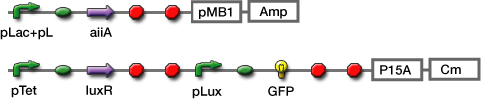
Experiments; Co-transformation; We constructed the circuit below. AiiA is placed on the high-copy plasmid under the control of Lac promoter. It was co-transformed with the LuxR/ Plux reporter on the p15A plasmid.
Communication; The resultant "AiiA/LuxR reporter" was co-cultured with LuxI sender and the fluorescence was monitored over time. As a control, we conducted the same experiment with LuxR reporter without AiiA plasmid.
Notes
Sender; LuxI plasmid transformed into E.coli strains (JW1908). Receiver; LuxR-gfp plasmid transformed into E.coli strains (JW1908). Culture/ Cndn.
#Both Sender and Receiver (+/- AiiA) were inoculated into small (2mL) culture and was shaken separately for 12h (at 37°C) #Inoculated into flesh media, shaken until cell density hit 2.0 in OD600 #Washed the cell and re-suspended. Cell density checked. #Mixed Sender and Receiver (Sender/Receiver 1000μl/1000μl). #Incubated at 30°C. #Time-chased the fluorescence (485nm(excitation) and 527nm(emission)) by gfp.
|
|
| [http://partsregistry.org/Part:BBa_S03623 BBa_S03623 (AHL sender)] |
|
|
Method
- Transformed Sender into E.coli strains(JW1908) and Receivers into E.coli strain(JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C 12h.
- Inoculated again at 37°C upto about OD600=2.0
- Washed them.
- Mixed them (Sender:Receiver=1000μl:1000μl).
- Incubated at 30°C.
- Measured intensity of green fluorescence at regular time intervals.
Result & Discussion
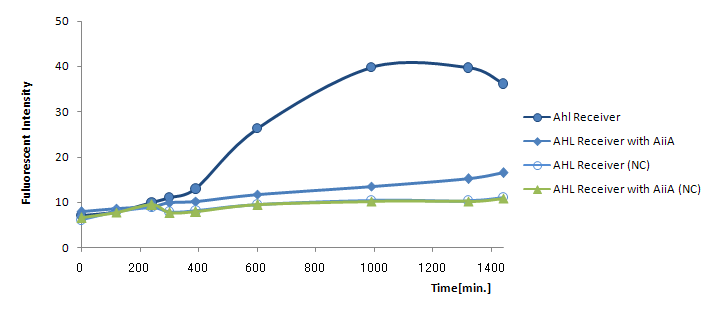
Results and discussion The co-expression of AiiA resulted in the drastic decrease in the fluorescence all through the experiment. It hasn't reached the endpoint even 24h after mixing. AiiA looks super-active and consume the most AHL molecule out; Obviously, the AHL activity is way too much.
On the other hand, we observed gradual increase in fluorescence over time. At least, the fluorescence from the co-culture was always above the negative control (without Lux-Sender). This indicate AiiA is not eating up the all signaling molecule. If we properly down-tune the AiiA activity (either by putting this gene into low-copy plasmid or by giving the low efficiency rbs), we should be able to see the time-delay.
References
- [http://www.jbc.org/cgi/content/full/279/14/13645 Wang et al.:Specificity and Enzyme Kinetics of the Quorum-quenching N-Acyl Homoserine Lactone Lactonase (AHL-lactonase).J. Biol. Chem.279(14),13645-13651,2004.]
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"