Team:Hawaii/Initial Synth. Oligo Assembly
From 2008.igem.org
(→Discussion) |
(→First attempt) |
||
| Line 27: | Line 27: | ||
==Results== | ==Results== | ||
===First attempt=== | ===First attempt=== | ||
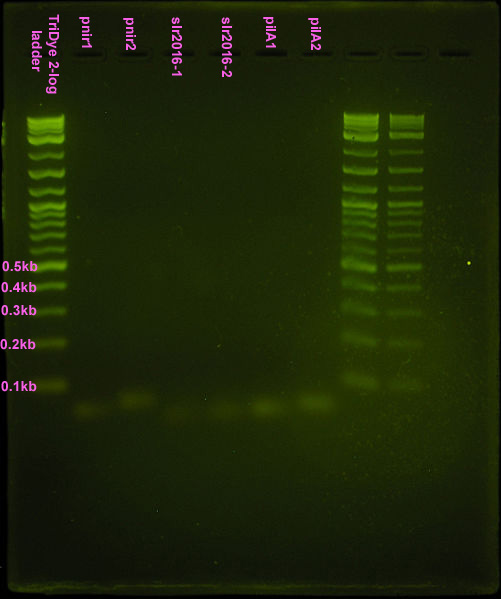
| - | [[Image:First assembly.jpg |left|thumb|200px|First assembly attempt. Ten microliters loaded into each well of | + | [[Image:First assembly.jpg |left|thumb|200px|First assembly attempt. Ten microliters loaded into each well of an EtBr stained ~3% agarose gel ran at 95V for 1 hr.]] |
Nice bands observed for annealed oligos. Bands appear to be the correct size (<100bp) but we can't tell if anneal worked (EtBr doesn't tell us if these are ssDNA or dsDNA bands). Smear is probably from loading way too much DNA (see discussion). Possible faint band for pnir ligation. No bands for slr2016 and pilA ligation, probably because way too much DNA was added for the ligation reaction. | Nice bands observed for annealed oligos. Bands appear to be the correct size (<100bp) but we can't tell if anneal worked (EtBr doesn't tell us if these are ssDNA or dsDNA bands). Smear is probably from loading way too much DNA (see discussion). Possible faint band for pnir ligation. No bands for slr2016 and pilA ligation, probably because way too much DNA was added for the ligation reaction. | ||
| + | ===Second attempt=== | ||
| + | Did a ligation using 1 μl of a 10<sup>-6</sup> dilution of each annealed product. Nothing was visible on the EtBr stained 4% agarose gel because not enough DNA was present. | ||
| + | ===Third attempt=== | ||
| + | [[Image:GK-Anneal_Test-SYBRSafe-UV.JPG|left|thumb|200px|Third ligation attempt. Ten microliters loaded into each well of an EtBr stained 4% agarose gel ran at 95V for 28 min.]] [[Image:GK-Anneal_Test-SYBRSafe-BlueLight.JPG|left|thumb|200px|SYBR Safe stained 2% agarose gel ran at 95V for 30 min. Ten microliters of a 10<sup>-1</sup> dilution of annealed product was loaded into each well.]] | ||
| + | Did a ligation using 10<sup>-2</sup> dilutions of annealed product. Faint bands were visible for the annealed products (10<sup>-2</sup> dilutions) but no bands were observed for the ligated products. Not enough DNA was present. Ran 10<sup>-1</sup> dilutions of annealed product on SYBR Safe stained 2% agarose gel. | ||
| + | ===Fourth attempt=== | ||
| + | Took 10 μl ligation reaction from first attempt and restriction digested with XbaI and PstI (1 μl each) in NEBuffer 3 (1 μl). Ran on EtBr stained 4% agarose gel alongside 10<sup>-1</sup> dilutions of annealed product. | ||
==Discussion== | ==Discussion== | ||
We need to dilute our annealed products before running them in a gel or attempting ligation. 20-125ng DNA (per lane) is good for running gels. One reaction using the NEB Quick Ligation Kit can ligate up to 180ng of DNA. | We need to dilute our annealed products before running them in a gel or attempting ligation. 20-125ng DNA (per lane) is good for running gels. One reaction using the NEB Quick Ligation Kit can ligate up to 180ng of DNA. | ||
Revision as of 05:17, 8 July 2008
Contents |
Protocol
Hybridization of Parts
- Mix:
- 3 μl 100 µM sense oligo
- 3 μl 100 µM anti-sense oligo
- 3 μl 10 x PNK (polynucleotide kinase) buffer
- 2 μl 10mM ATP
- 2 μl T4 polynucleotide kinase (PNK)
- 17 μl distilled water
- Total volume = 30 μl
- Incubate at 37C for 1.5 hours.
- Add 4 μl 0.5 M NaCl.
- Place in boiling water bath for 2 min., then remove water bath from the heat source and allow the reaction (still in the water bath) to cool to room temperature (approx. 30 minutes)
- Reference: Pam Silver Lab. [http://openwetware.org/wiki/Silver:_Oligonucleotide_Inserts| Oligonucleotide Inserts.]
DNA Ligation
- Combine 4 ul of construct one with 4 ul of construct 2.
- Add 1 ul of nanopure H20.
- Add 10 ul of 2x quick ligation reaction Buffer. In order to avoid shearing the DNA, mix by resuspending very slowly.
- Add 1 ul of Quick T4 DNA ligase and mix thoroughly by resuspending very slowly.
- Incubate at room temperature for 5 minutes.
- Cool on ice then transform, or store at -20oC
- Reference: Quick Ligation Kit from NEB.
Results
First attempt
Nice bands observed for annealed oligos. Bands appear to be the correct size (<100bp) but we can't tell if anneal worked (EtBr doesn't tell us if these are ssDNA or dsDNA bands). Smear is probably from loading way too much DNA (see discussion). Possible faint band for pnir ligation. No bands for slr2016 and pilA ligation, probably because way too much DNA was added for the ligation reaction.
Second attempt
Did a ligation using 1 μl of a 10-6 dilution of each annealed product. Nothing was visible on the EtBr stained 4% agarose gel because not enough DNA was present.
Third attempt
Did a ligation using 10-2 dilutions of annealed product. Faint bands were visible for the annealed products (10-2 dilutions) but no bands were observed for the ligated products. Not enough DNA was present. Ran 10-1 dilutions of annealed product on SYBR Safe stained 2% agarose gel.
Fourth attempt
Took 10 μl ligation reaction from first attempt and restriction digested with XbaI and PstI (1 μl each) in NEBuffer 3 (1 μl). Ran on EtBr stained 4% agarose gel alongside 10-1 dilutions of annealed product.
Discussion
We need to dilute our annealed products before running them in a gel or attempting ligation. 20-125ng DNA (per lane) is good for running gels. One reaction using the NEB Quick Ligation Kit can ligate up to 180ng of DNA.
 "
"