Team:University of Lethbridge/Notebook/Project2July
From 2008.igem.org
(→Roxanne, Selina: -added gel pics and corrected labelling) |
(→Roxanne, Selina) |
||
| Line 44: | Line 44: | ||
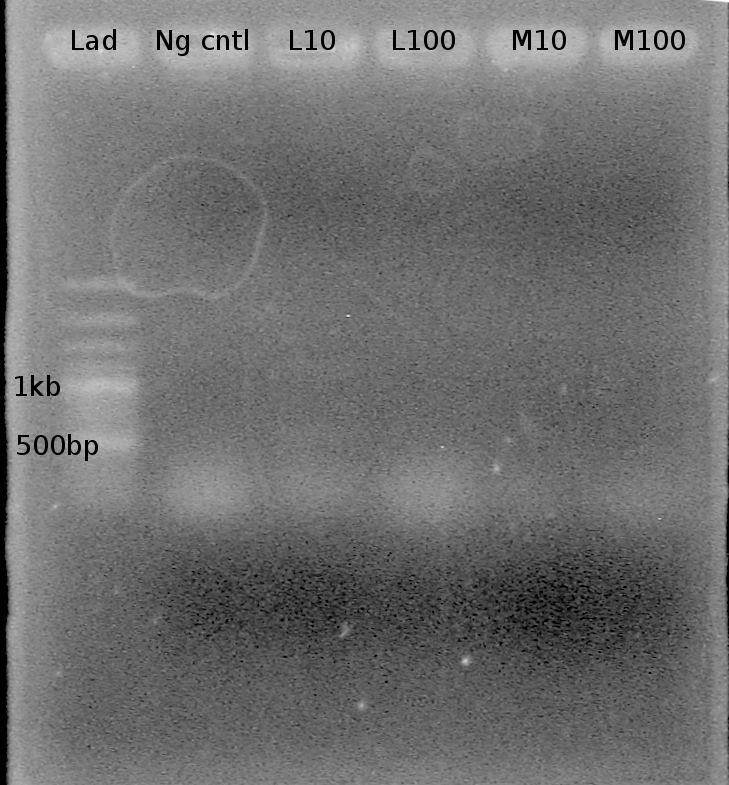
Results? Bands present in all lanes! (all less than 100 bp) However, unsure if the bands are primer bands or Riboswitch sequence DNA bands. Nathan to run a 3% Agarose gel tonight to try and get better resolution. | Results? Bands present in all lanes! (all less than 100 bp) However, unsure if the bands are primer bands or Riboswitch sequence DNA bands. Nathan to run a 3% Agarose gel tonight to try and get better resolution. | ||
| + | |||
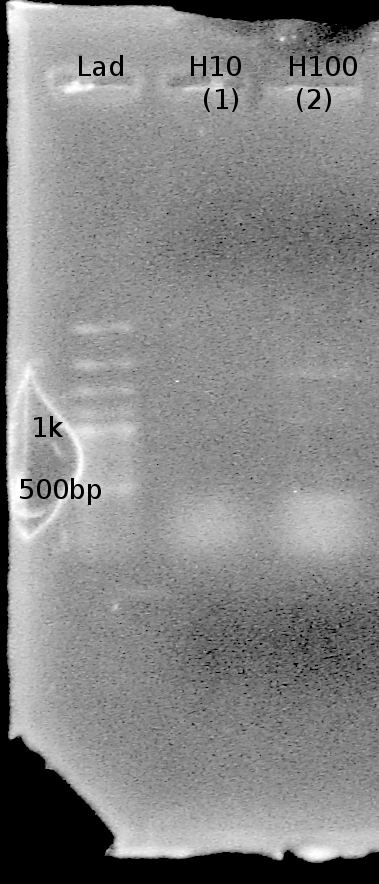
| + | note: Double bands present in this gel as well. Two bands at ~1kb and 1.5 kb are present in lanes L10 and H10. | ||
[[Image:08Jul28_RSPCR.jpg|250 px]] | [[Image:08Jul28_RSPCR.jpg|250 px]] | ||
[[Image:08Jul28_RSPCR2.jpg|125 px]] | [[Image:08Jul28_RSPCR2.jpg|125 px]] | ||
Revision as of 18:59, 30 July 2008
Contents |
July 28, 2008
Nathan Puhl, Roxanne
Objective: Amplify Riboswitch sequence from pTopp plasmid using PCR Reaction
PCR reaction setup:
-Enzyme = Phusion -Template = pTopp plasmid, dilution series of 1:10, 1:100 and (-) control -Buffer = HF Phusion buffer -Primers = IDT, designed & shipped July 16/08 -Total # of reactions = 20
PCR cycle conditions (programmed into Brent's PCR machine under "iGEM":
1. Initial denaturation @ 98 C for 30 sec (1 cycle) 2a. Denaturation @ 98 C for 30 sec (35 cycles for step #2) 2b. Annealing via gradient for 15 sec
45.0 C to 53.0 C
2c. Extension @ 72 C for 30 sec 3. Final extension @ 72 C for 7 mins (1 cycle) 4. Hold at 4 C
July 29, 2008
Roxanne, Selina
Objective: Visualize whether any Ribswitch PCR reactions (July 28) were successful.
Ran 1% TAE agarose gel at 100 V for 30 mins
Loaded 5 uL sample, 1 uL ladder
Samples:
Gel #1:
- Lane 1: 100 bp ladder - Lane 2: Neg cntl - Lanes 3-5: 1:10 template dilution, annealing temps (45.0 C, 49.0 C, 53.0 C)
Gel #2:
- Lane 1: 100 bp ladder - Lanes 2-3: 1:100 template dilution, annealing temps (45.0 C, 49.0 C)
Results? Bands present in all lanes! (all less than 100 bp) However, unsure if the bands are primer bands or Riboswitch sequence DNA bands. Nathan to run a 3% Agarose gel tonight to try and get better resolution.
note: Double bands present in this gel as well. Two bands at ~1kb and 1.5 kb are present in lanes L10 and H10.
 "
"