Team:Newcastle University/Conclusions
From 2008.igem.org
Riachalder (Talk | contribs) |
Riachalder (Talk | contribs) |
||
| Line 5: | Line 5: | ||
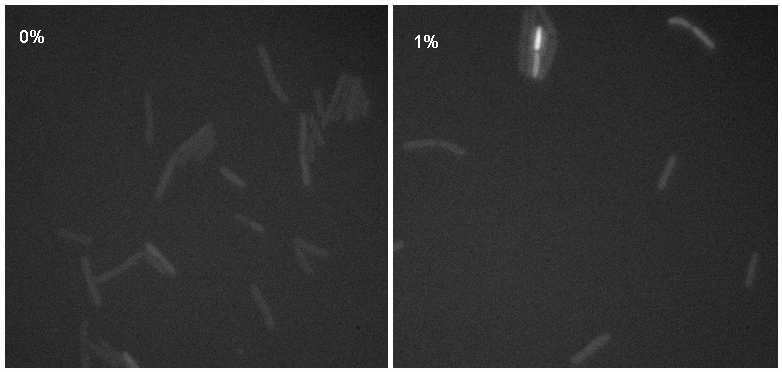
[[Image:Montage.jpg|thumb|right|300px|Microscopy results of iGEMgfp upon 1% induction by subtilin]] | [[Image:Montage.jpg|thumb|right|300px|Microscopy results of iGEMgfp upon 1% induction by subtilin]] | ||
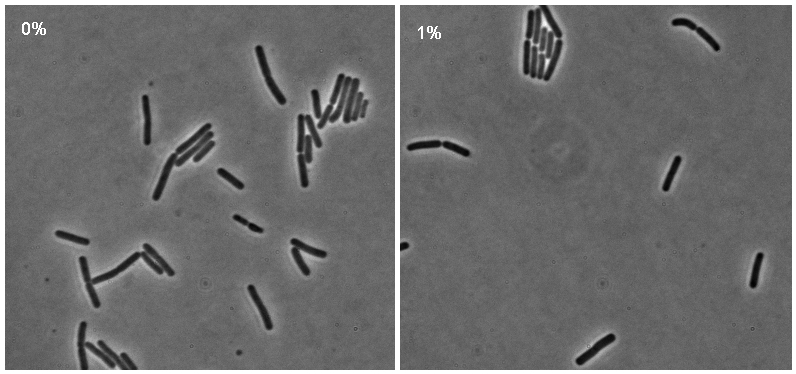
[[Image:Montage_pc.jpg|thumb|right|300px|Microscopy results of iGEMcherry upon 1% induction by subtilin]] | [[Image:Montage_pc.jpg|thumb|right|300px|Microscopy results of iGEMcherry upon 1% induction by subtilin]] | ||
| - | + | ||
| - | + | ||
<html> | <html> | ||
</div><!--maincontent --> | </div><!--maincontent --> | ||
</html> | </html> | ||
| - | To analyse the results of the wet lab transformations of the inserts into ''B. subtilis'', we used two methods: microscopy and flow cytometry | + | To analyse the results of the wet lab transformations of the inserts into ''B. subtilis'', we used two methods: [[microscopy]] and [[flow cytometry]]. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Microscopy work done on 08.09.08 showed a possible difference in the brightness of the iGEMgfp fluorescent cells (brighter in 10% subtilin-induced cells than in 0% subtilin-induced cells). However, there was little difference in the numbers of cells that fluoresced between the two cultures. | |
| - | + | There was no significant difference in either the number of fluorecent cells or their brightness between the 10% subtilin-induced and the 0% subtilin-induced iGEMcherry cells. Both cultures fluoresced very poorly. It was suggested that this could be due to the ''mCherry'' being inherently weak in its fluorecence. | |
| + | Flow cytometry was performed on the iGEMgfp culture to determine whether the microscopy work did actually show a reporter gene induction by subtilin. This revealed a mean fluorescence of 7.70 counts for 0% subtilin induction, 14.77 counts for 1% subtilin induction and 21.95 counts for 10% subtilin induction. The dramatic increase between each of the measurements suggests that the ncl08 2 component spaRK system + ''gfp'' has inserted correctly into the ''B. subtilis'' chromosomsal DNA and is inducible by subtilin. The fact that two distinct cell populations can be observed in the 10% subtilin induced culture shows that the supernatent added to the cells does indeed contain functional subtilin - one of these populations are the cells that are lysing as a result of the subtilin antibiotic. | ||
<div id="sidebar"> | <div id="sidebar"> | ||
Revision as of 13:09, 19 September 2008
Newcastle University
GOLD MEDAL WINNER 2008
| Home | Team | Original Aims | Software | Modelling | Proof of Concept Brick | Wet Lab | Conclusions |
|---|
Results
To analyse the results of the wet lab transformations of the inserts into B. subtilis, we used two methods: microscopy and flow cytometry.
Microscopy work done on 08.09.08 showed a possible difference in the brightness of the iGEMgfp fluorescent cells (brighter in 10% subtilin-induced cells than in 0% subtilin-induced cells). However, there was little difference in the numbers of cells that fluoresced between the two cultures.
There was no significant difference in either the number of fluorecent cells or their brightness between the 10% subtilin-induced and the 0% subtilin-induced iGEMcherry cells. Both cultures fluoresced very poorly. It was suggested that this could be due to the mCherry being inherently weak in its fluorecence.
Flow cytometry was performed on the iGEMgfp culture to determine whether the microscopy work did actually show a reporter gene induction by subtilin. This revealed a mean fluorescence of 7.70 counts for 0% subtilin induction, 14.77 counts for 1% subtilin induction and 21.95 counts for 10% subtilin induction. The dramatic increase between each of the measurements suggests that the ncl08 2 component spaRK system + gfp has inserted correctly into the B. subtilis chromosomsal DNA and is inducible by subtilin. The fact that two distinct cell populations can be observed in the 10% subtilin induced culture shows that the supernatent added to the cells does indeed contain functional subtilin - one of these populations are the cells that are lysing as a result of the subtilin antibiotic.
 "
"