Team:Heidelberg/Notebook/Killing II/2ndweek
From 2008.igem.org
(Difference between revisions)
(→General) |
|||
| Line 313: | Line 313: | ||
===General=== | ===General=== | ||
*Seminar on Synthetic Biology | *Seminar on Synthetic Biology | ||
| - | [[Team:Heidelberg/Notebook/Killing_II/ | + | [[Team:Heidelberg/Notebook/Killing_II/3rdweek | go to 3<sup>rd</sup> week]] |
Revision as of 08:39, 27 October 2008


2nd week
Contents |
Monday 08/11/2008
Colicin Receiver
Cloning
- Inoculation of cultures Receiver BBa_F2621, Receiver BBa_F2622 and GFP-Receiver BBa_T9002 for Miniprep and Glycerolstocks (LB) as well as in M9 (tests).
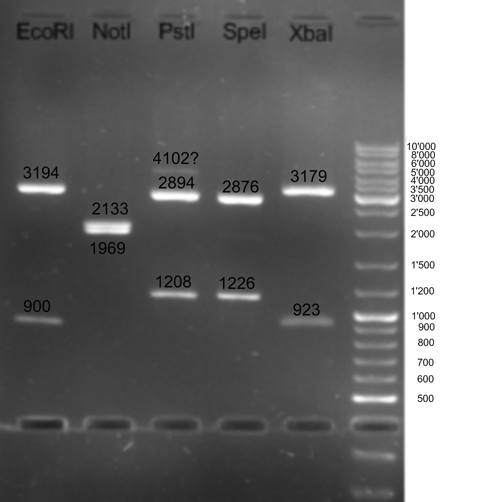
- Digestion of BBa_T9002 to control which restriction sites are available in MCS of pSB1A3: 1.5 - 2 h -> 37 °C 10 min -> 65 °C
5.0 µl 10x NEBuffer 3 (NEB) 5.0 µl BSA 10x (NEB) 28.0 µl H2O ------- 50.0 µl
2.0 µl SpeI (NEB) 4.0 µl BglI (Fermentas 10.0 µl Miniprep DNA 5.0 µl 10x NEBuffer 2 5.0 µl BSA 10x (NEB) 26.0 µl H2O ------- 50.0 µl
2.0 µl XbaI (Fermentas) 4.0 µl BglI (Fermentas) 10.0 µl Miniprep DNA 10.0 µl Tangobuffer (Fermentas) 5.0 µl BSA 10x (NEB) 26.0 µl H2O ------- 50.0 µl
4.0 µl EcoRI (Fermentas) 4.0 µl BglI (Fermentas) 10.0 µl Miniprep DNA 10.0 µl Buffer Orange ( 5.0 µl BSA 10x (NEB) 24.0 µl H2O ------- 50.0 µl
4.0 µl PstI (Fermentas) 4.0 µl BglI (Fermentas) 10.0 µl Miniprep DNA 5.0 µl Buffer Orange (Fermentas) 5.0 µl BSA 10x (NEB) 28.0 µl H2O ------- 50.0 µl
- Transformation of BBa_F2621 and BBa_F2622 with Registry protocol
General
- Matlab course + evaluation
Tuesday 08/12/2008
Colicin Receiver
Cloning
- Results Receiver T9002 digestion: All restriction sites of the MCS are still available
- Miniprep of BBa_F2622 and BBa_I2621: eluted in 30 µl H2O
Activity Test
- Dilution of ONC 1:50
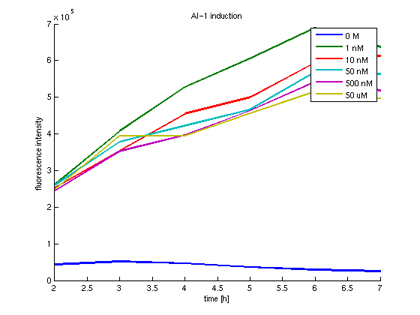
- Activation of BBa_T9002 with AHL:
c1 = 1 nM c2 = 10 nM c3 = 50 nM c4 = 500 nM c5 = 50 µM
- Measurement in Tecan well plate reader hourly (7h):
- black plate: OD & GFP
- transparent plate: OD
Sender part
Cloning
- Miniprep of BBa_F1610 and BBa_I15030: eluted in 30 µl H2O
Activity Test
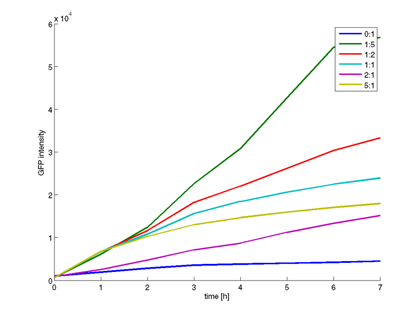
- Measurement of sender BBa_F1610 and amplifier BBa_I15030 activity with BBa_T9002 hourly. Incubation at 37 °C.
- Sender/Amplifier - Receiver Ratios (constant receiver ratio):
S/A - R 1 - 5 1 - 2 1 - 1 2 - 1 5 - 1
Wednesday 08/13/2008
Colicin Receiver
Cloning
- pColE9-J arrived from Kleanthous Lab University of York, UK
- Transformation of BBa_T9002, BBa_F2621, BBa_F2622 and pColE9-J into MG1655 (Transformation protocol Chris)
- Control digestion of parts received from iGEM HQ: BBa_ F2621 (SfcI) and BBa_F2622 (SfcI); 37 °C
10 µl DNA 5 µl Buffer 10x 2 µl Enzyme 5 µl BSA 10x 28 µl H2O ----- 50 µl
- Results: Many fragments which cannot be differentiated.
Activity Test
- Data evaluation with Matlab
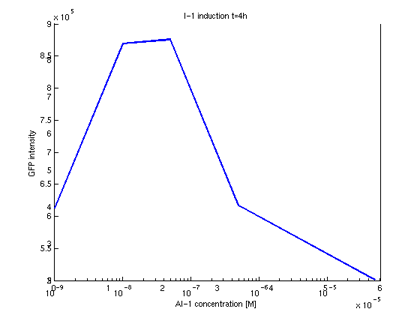
- Quantitative measurement of GFP expression from receiverpart T9002 induced by AI-1
- Plot of GFP expression against time for different AI-1 concentrations
- Plot of GFP expression against AI-1 concentrations for t=4h
- Plot of GFP expression against time for different AI-1 concentrations
Sender part
Cloning
- Transformation of F1610 into MG1655 (Transformation protocol Chris)
- Control digestion of parts received from iGEM HQ: BBa_F1610 (DraI) and BBa_I15030 (BsaBI); 37 °C (DraI) and 65 °C (BsaBI)
10 µl DNA 5 µl Buffer 10x 2 µl Enzyme 5 µl BSA 10x 28 µl H2O ----- 50 µl
- Results: Many fragments which cannot be differentiated.
Activity Test
- Data evaluation with Matlab
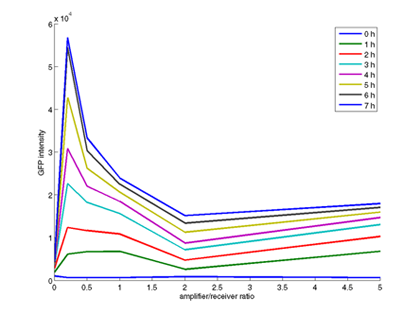
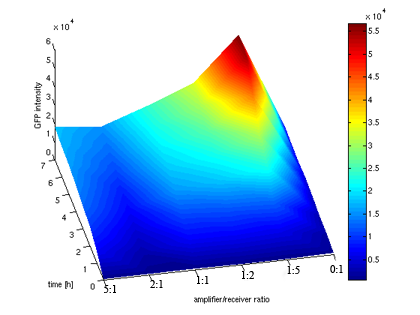
- Quantitative measurement of GFP expression from Receiverpart T9002 induced by amplifierpart I15030
- Plot of GFP expression against time for different amplifier/receiver ratios
- Plot of GFP expression against amplifier/receiver ratios for different times
- Surfaceplot of GFP expression against amplifier/receiver ratios and time
- Plot of GFP expression against time for different amplifier/receiver ratios
Thursday 08/14/2008
Colicin Receiver
Cloning
- Inoculation of liquid cultures from transformation of wednesday: BBa_F2621, BBa_F2622, BBa_T9002 and pColE9-J in LB-media with antibiotics. Incubation at 37 °C ON.
Sender part
Cloning
- Inoculation of liquid cultures from transformation of wednesday: BBa_F1610 and BBa_I15030 in LB-media with antibiotics.
Activity Test
- Inoculation of BBa_T9002 and BBa_F1610 in M9-Media
General
- Preperation for Team Meeting:
- Presentation of colicin project of tje NTU Singapore
- Presentation of results and future prospects
Friday 08/15/2008
Colicin Receiver
Cloning
- Glycerolstocks of BBa_F2621, BBa_F2622, BBa_T9002 and pColE9-J cultures.
1 ml culture + 150 µl 80% Glycerol vortex briefly 30 min Incubation at RT -> -80 °C freezer
- Minipreps of BBa_F2621, BBa_F2622, BBa_T9002 and pColE9-J cultures.
- Primer Design colicinE1:
- fwd on colicin E1 gene with BamHI site (XbaI)
- rv on colicin E1 gene with BamHI site (SpeI)
- rv on immunity gene with (SpeI)
- rv on kil gene with SpeI
- rv in front of SOS-Box (SpeI)
- fwd immunity gene (XbaI)
- fwd kil gene (XbaI)
- Primer Design colicinE9:
- fwd on colicinE9 with BamHI (XbaI)
- rv on canonical lysis gene (SpeI)
- rv on ColE9 (SpeI)
Activity Test
- AI-1 measurement (see protocol Tuesdaysday for details) 8h
Sender part
Cloning
- Glycerolstocks of BBa_F1619 & BBa_I15030 cultures.
1 ml culture + 150 µl 80% Glycerol vortex briefly 30 min Incubation at RT -> -80 °C freezer
- Minipreps of BBa_F1619 & BBa_I15030 cultures.
Activity Tests
- Amplifier measurement (see protocol Tuesdaysday for details) 8h: but we used a 1:25 dilution of Receiver cells
General
- Seminar on Synthetic Biology
 "
"