Team:Imperial College/Genetic Circuit
From 2008.igem.org
| Line 5: | Line 5: | ||
{{Imperial/Box1|Modelling Constitutive Gene Expression| | {{Imperial/Box1|Modelling Constitutive Gene Expression| | ||
| + | |||
A simple [[Imperial_College/Courses/Spring2008/Synthetic_Biology/Computer_Modelling_Practicals/Practical_2 | synthesis-degradation model]] is assumed for the modelling of the expression of a protein under the control of a constitutive promoter, with the same model assumed for all four [[Team:Imperial_College/Cloning_Strategy | promoter-RBS constructs]]. The synthesis-degradation model assumes a steady state level of mRNA. | A simple [[Imperial_College/Courses/Spring2008/Synthetic_Biology/Computer_Modelling_Practicals/Practical_2 | synthesis-degradation model]] is assumed for the modelling of the expression of a protein under the control of a constitutive promoter, with the same model assumed for all four [[Team:Imperial_College/Cloning_Strategy | promoter-RBS constructs]]. The synthesis-degradation model assumes a steady state level of mRNA. | ||
| - | + | [[Image:Eq1.png]] | |
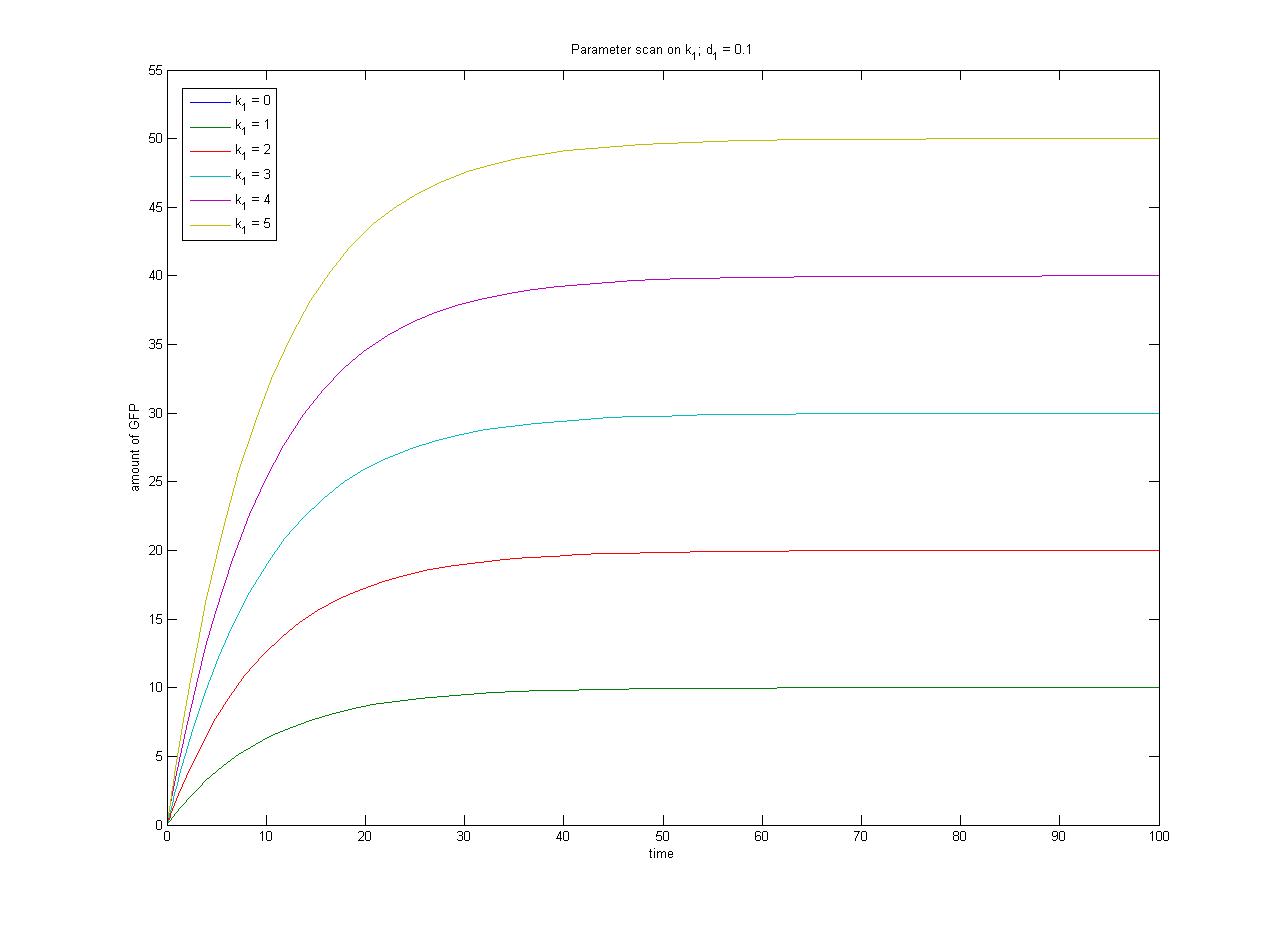
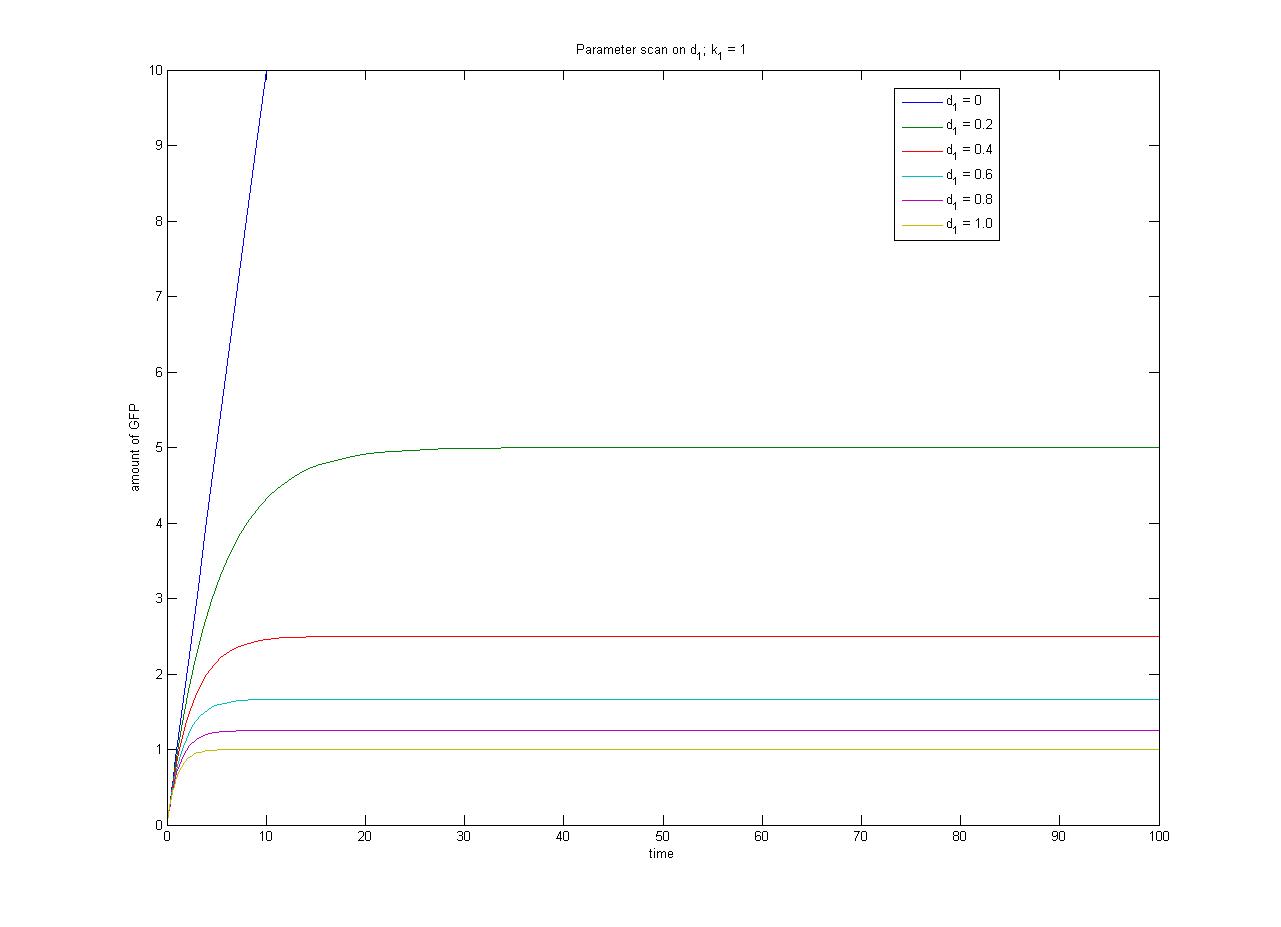
In this case, [protein] represents the concentration of GFP, k1 represents the rate of sythesis and d1 represents the degradation rate. | In this case, [protein] represents the concentration of GFP, k1 represents the rate of sythesis and d1 represents the degradation rate. | ||
| Line 15: | Line 16: | ||
<br> | <br> | ||
| - | We can also solve this ODE analytically. Consider the steady-state behaviour of [protein]. | + | We can also solve this ODE analytically. |
| + | |||
| + | [[Image:Eq_analytical_soln_constitutive_expression.png]] | ||
| + | |||
| + | Consider the steady-state behaviour of [protein]. | ||
| - | + | [[Image:Eq2.png]] | |
| - | + | The relationship between the steady-state protein concentration and the parameters can be seen in the parameter scan graphs on the right. | |
From the wetlab experiments it is likely that we will obtain steady-state data for each of the four promoter-RBS constructs. If we assume the same rate of degradation of GFP in each case, we can have some measure of the relative rate of transcription through each promoter which will help us with the selection of the most appropriate promoter to use for Phase 2. In order to obtain an absolute measure of transcription (as opposed to a relative measure of transcriptional strength) we require constitutive expression in terms of molecules per cell (as opposed to fluorescence in arbitrary units). | From the wetlab experiments it is likely that we will obtain steady-state data for each of the four promoter-RBS constructs. If we assume the same rate of degradation of GFP in each case, we can have some measure of the relative rate of transcription through each promoter which will help us with the selection of the most appropriate promoter to use for Phase 2. In order to obtain an absolute measure of transcription (as opposed to a relative measure of transcriptional strength) we require constitutive expression in terms of molecules per cell (as opposed to fluorescence in arbitrary units). | ||
Revision as of 18:22, 27 October 2008
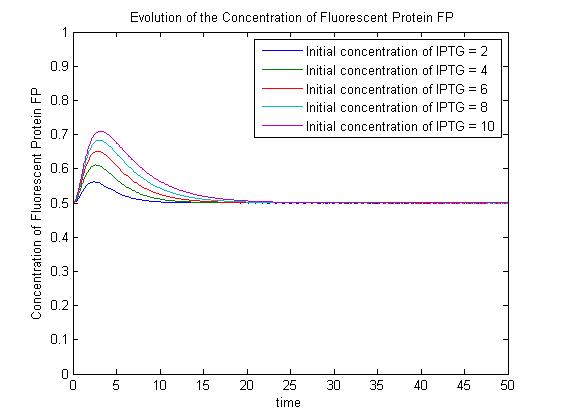
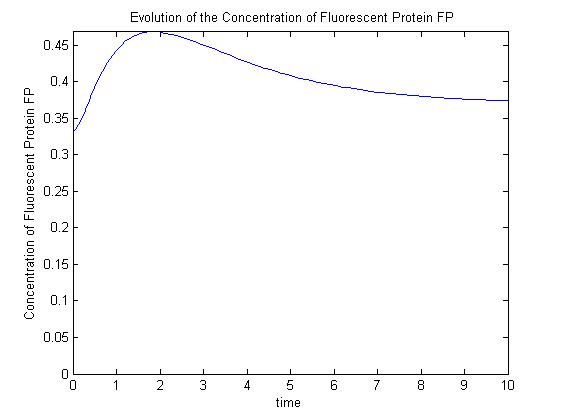
Modelling the Genetic Circuit
--Mabult 14:44, 27 October 2008 (UTC) Cut this part out and move it into Pridence's section (simple repression Model)
|
|||||||||||||||||||||||||
 "
"