Team:Bay Area RSI/Project
From 2008.igem.org
(→Targeting Infarcted Cardiac Tissue) |
(→Project Overview) |
||
| Line 45: | Line 45: | ||
| - | == ''' | + | == '''Abstract''' == |
| - | Every year over 1.2 million people suffer myocardial infarction. The resulting heart damage requires new approaches for effective repair. Stem cell therapies provide hope. However none of the stem cell therapies currently in clinical trials addresses the need for efficient stem cell targeting to cardiac tissue or the need to replace efficiently dead tissue with new cardiomyocytes. To address these problems, we have built several genetic circuits that work sequentially to repair the heart. First, we have built an inducible differentiation circuit that closely resembles the endogenous differentiation pathway, to program cells to become cardiomyocytes. Second, we have built circuits that use the extracellular domains of chimeric proteins to target cells to damaged cardiac tissue. Upon binding, novel receptor-coupled intein-mediated signaling domains activate effector genes that then aid in integration, inhibition of cell death, and the alteration of the tissue microenvironment. | + | Every year over 1.2 million people suffer myocardial infarction (MI). The resulting heart damage requires new approaches for effective repair. Stem cell therapies provide hope. However none of the stem cell therapies currently in clinical trials addresses the need for efficient stem cell targeting to cardiac tissue or the need to replace efficiently dead tissue with new cardiomyocytes. To address these problems, we have built several genetic circuits that work sequentially to repair the heart. First, we have built an inducible differentiation circuit that closely resembles the endogenous differentiation pathway, to program cells to become cardiomyocytes. Second, we have built circuits that use the extracellular domains of chimeric proteins to target cells to damaged cardiac tissue. Upon binding, novel receptor-coupled intein-mediated signaling domains activate effector genes that then aid in integration, inhibition of cell death, and the alteration of the tissue microenvironment. |
| + | |||
| + | == '''Introduction''' == | ||
| + | In 2007 the RSI Bay Area Consortium Team designed and engineered novel methods of targeting damaged cardiomyocytes. Since then, we have shown that our targeting circuit for damaged cardiomyocytes binds and relays effectors in rat cardiomyoblasts. To complement this circuit, the 2008 RSI Bay Area Consortium Team chose to create an efficient inducible method for the differentiation of cardiomyocytes. Together, the two circuits resolve many of the problems associated with the current therapies for MI patients. | ||
== '''''Targeting Infarcted Cardiac Tissue'''''== | == '''''Targeting Infarcted Cardiac Tissue'''''== | ||
Revision as of 21:18, 28 October 2008
| You can write a background of your team here. Give us a background of your team, the members, etc. Or tell us more about something of your choosing. | |
|
Tell us more about your project. Give us background. Use this is the abstract of your project. Be descriptive but concise (1-2 paragraphs) | |
| Team Example 2 |
| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Notebook |
|---|
(Or you can choose different headings. But you must have a team page, a project page, and a notebook page.)
Abstract
Every year over 1.2 million people suffer myocardial infarction (MI). The resulting heart damage requires new approaches for effective repair. Stem cell therapies provide hope. However none of the stem cell therapies currently in clinical trials addresses the need for efficient stem cell targeting to cardiac tissue or the need to replace efficiently dead tissue with new cardiomyocytes. To address these problems, we have built several genetic circuits that work sequentially to repair the heart. First, we have built an inducible differentiation circuit that closely resembles the endogenous differentiation pathway, to program cells to become cardiomyocytes. Second, we have built circuits that use the extracellular domains of chimeric proteins to target cells to damaged cardiac tissue. Upon binding, novel receptor-coupled intein-mediated signaling domains activate effector genes that then aid in integration, inhibition of cell death, and the alteration of the tissue microenvironment.
Introduction
In 2007 the RSI Bay Area Consortium Team designed and engineered novel methods of targeting damaged cardiomyocytes. Since then, we have shown that our targeting circuit for damaged cardiomyocytes binds and relays effectors in rat cardiomyoblasts. To complement this circuit, the 2008 RSI Bay Area Consortium Team chose to create an efficient inducible method for the differentiation of cardiomyocytes. Together, the two circuits resolve many of the problems associated with the current therapies for MI patients.
Targeting Infarcted Cardiac Tissue
C-Reactive Protein Receptor Intein Mediated Signaling Circuit
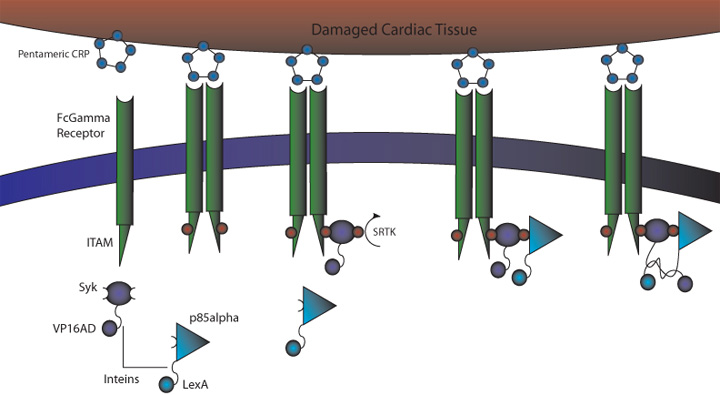 Continuation from last year
Circuit overview
construct image
Continuation from last year
Circuit overview
construct image
FCGamma Receptor Binds Immobilised CRP on Damaged Cardiomyocytes In Vitro
description picture picture exp
CRP activates GFP signaling upon FCGamma Binding
picture picture exp
Generation of Clonal lines of FCGamma+ rat H9C2 Cardiomyocytes
pic + explanation
Effectors To Aid in integration, anti-apoptosis, and the alteration of the tissue microenvironment
Overview Construct image pics from slides
CRP Targeting Circuit Concerns
Future Directions
Sensor sensitivity-> , avidity, affinity More universal receptor --> 2nd gen SCFV to target other tissue
Cardiomyocyte Differentiation Circuit
endogenous pathways pathway overview construct image + explanation data
Differentiation Circuit Concerns
CA, efficiency, transdiff,
Future Directions
inducible, non-SC progenitor, native cardiofiobroblast
Use As a Novel Therapy For MI Patients
Current therapies and problems Diff types of MI and need for better solution Therpeautic approach with combined circuitry use in tandem, synergistic application
 "
"

