Team:Cambridge/Signalling/Lab Work
From 2008.igem.org
(→RBS screening) |
(→Backbone for ligation) |
||
| Line 650: | Line 650: | ||
- Plasmid miniprep | - Plasmid miniprep | ||
| + | |||
| + | =September, 12th= | ||
| + | |||
| + | ==RBS screening (single digest)== | ||
| + | |||
| + | - Plasmid miniprep 6 colonies of RBS S and 6 colonies of RBS W (without endo wash buffer) | ||
| + | |||
| + | - Nanodrop | ||
| + | |||
| + | {|class="wikitable" style="text-align:center" border="1" | ||
| + | |- | ||
| + | ! product !! concentration (ng/μL) !! 260/280 !! quantity of DNA to add to have about 300ng (μL) !! added SDW for single digest (μL) | ||
| + | |- | ||
| + | | RBS W1 || 57.1 || 1.57 || 6 || 11 | ||
| + | |- | ||
| + | | RBS W3 || 54.1 || 1.53 || 6 || 11 | ||
| + | |- | ||
| + | | RBS W5 || 15.1 || 1.85 || 17 || 0 | ||
| + | |- | ||
| + | | RBS W 7|| 21.8 || 1.66 || 15 || 2 | ||
| + | |- | ||
| + | | RBS W9|| 21.8 || 1.78 || 15 || 2 | ||
| + | |- | ||
| + | | RBS W11 || 13.8 || 1.73 || 17 || 0 | ||
| + | |- | ||
| + | | RBS S2 || 19.5 || 1.7 || 15 || 2 | ||
| + | |- | ||
| + | | RBS S4 || 82.13 || 2.57 || 4 || 13 | ||
| + | |- | ||
| + | | RBS S6 || 44.7 || 1.64 || 7 || 10 | ||
| + | |- | ||
| + | | RBS S8 || 104.0 || 1.48 || 3 || 14 | ||
| + | |- | ||
| + | | RBS S10 || 13.1 || 1.82 || 17 || 0 | ||
| + | |- | ||
| + | | RBS S11 || 20.1 || 1.73 || 15 || 2 | ||
| + | |} | ||
| + | |||
| + | - Single digest : with EcoRI and XbaI, add SDW and DNA (according to the previous table, 300mg of DNA), 2μL of fast digest buffer, and 1μL of enzyme | ||
| + | |||
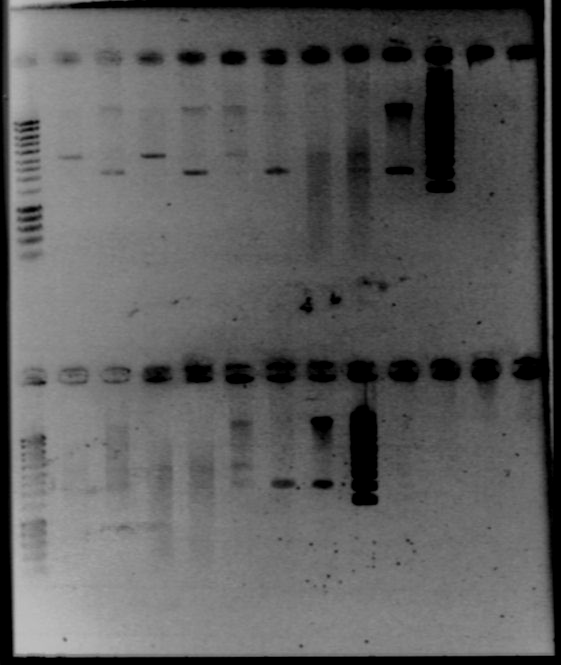
| + | - Gel1 | ||
| + | |||
| + | *Lane2 : HyperladderI | ||
| + | *Lane3 : RBS W1 cut with EcoRI | ||
| + | *Lane4 : RBS W1 cut with XbaI | ||
| + | *Lane5 : RBS W3 cut with EcoRI | ||
| + | *Lane6 : RBS W3 cut with XbaI | ||
| + | *Lane7 : RBS W7 cut with EcoRI | ||
| + | *Lane8 : RBS W7 cut with XbaI | ||
| + | *Lane9 : RBS W9 cut with EcoRI | ||
| + | *Lane10 : RBS W9 cut with XbaI | ||
| + | *Lane11 : RBS W9 uncut | ||
| + | *Lane12 : supercoiled ladder | ||
| + | |||
| + | |||
| + | - Gel2 | ||
| + | |||
| + | *Lane2 : HyperladderI | ||
| + | *Lane3 : RBS S4 cut with EcoRI | ||
| + | *Lane4 : RBS S4 cut with XbaI | ||
| + | *Lane5 : RBS S6 cut with EcoRI | ||
| + | *Lane6 : RBS S6 cut with XbaI | ||
| + | *Lane7 : RBS S8 cut with EcoRI | ||
| + | *Lane8 : RBS S8 cut with XbaI | ||
| + | *Lane9 : RBS S11 cut with EcoRI | ||
| + | *Lane10 : RBS S11 cut with XbaI | ||
| + | *Lane11 : RBS S4 uncut | ||
| + | *Lane12 : supercoiled ladder | ||
| + | |||
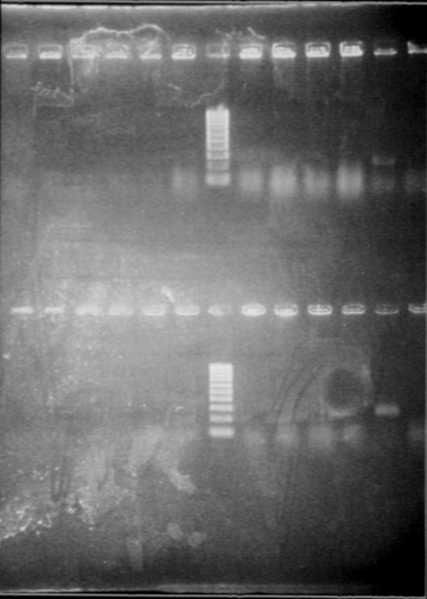
| + | *Result | ||
| + | |||
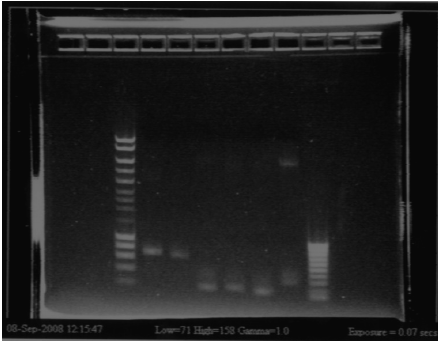
| + | [[Image:gel21092pcr.gif|250px|center]] | ||
| + | |||
| + | The size of our plasmid is about 3000bp (ok on the gel) | ||
| + | |||
| + | *RBS W | ||
| + | |||
| + | - W1 with EcoRI : cut | ||
| + | |||
| + | - W1 with XbaI : uncut, self ligation | ||
| + | |||
| + | - W3 with EcoRI : cut | ||
| + | |||
| + | - W3 with XbaI : uncut, self ligation | ||
| + | |||
| + | - W7 with EcoRI : impossible to see | ||
| + | |||
| + | - W7 with XbaI : uncut, self ligation | ||
| + | |||
| + | - W9 with EcoRI : cut | ||
| + | |||
| + | - W9 with XbaI : uncut, self ligation | ||
| + | |||
| + | *RBS S | ||
| + | |||
| + | - S4 with EcoRI : cut | ||
| + | |||
| + | - S4 with XbaI : uncut, self ligation | ||
| + | |||
| + | - S6 with EcoRI : cut | ||
| + | |||
| + | - S6 with XbaI : 2 band, this plasmid is partially cut!!! Transformation with RBS S | ||
| + | |||
| + | - S8 with EcoRI : cut | ||
| + | |||
| + | - S8 with XbaI : uncut, self ligation | ||
| + | |||
| + | - S11 with EcoRI : cut | ||
| + | |||
| + | - S11 with XbaI : uncut, self ligation | ||
| + | |||
| + | ==Double check of RBS S6 and stock== | ||
| + | |||
| + | - Grow RBS S6 in LB with Cm35 | ||
| + | |||
| + | ==Check more RBS W== | ||
| + | |||
| + | - Grow RBS W2, 4, 6, 8, 10 in 10mL of LB with antibiotic | ||
| + | |||
| + | ==Check PCR products== | ||
| + | |||
| + | - Gel (2μL of each product) | ||
| + | |||
| + | *Lane1 : λ ladder | ||
| + | *Lane2 : agrA | ||
| + | *Lane3 : agrB | ||
| + | *Lane4 : agrC | ||
| + | *Lane5 : rep | ||
| + | *Lane6 : pSB4C5 1 | ||
| + | *Lane7 : pSB4C5 2 | ||
| + | *Lane8 : Pxyl | ||
| + | *Lane9 : Ppac | ||
| + | *Lane10 : RBS S | ||
| + | *Lane11 : RBS W | ||
| + | *Lane12 : HyperladderI | ||
| + | |||
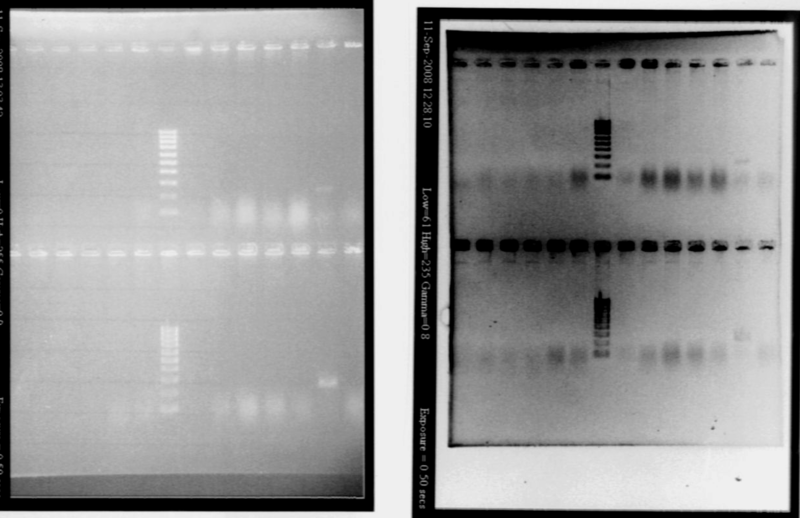
| + | *Results | ||
| + | |||
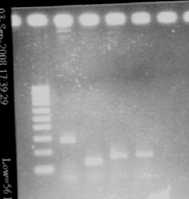
| + | [[Image:gel21091pcr.gif|200px|center]] | ||
| + | |||
| + | - agr B : 2 bands? | ||
| + | |||
| + | - agrC : ok | ||
| + | |||
| + | - rep : small band but good size | ||
| + | |||
| + | - backbones : ok | ||
| + | |||
| + | - promoters : ok | ||
| + | |||
| + | - RBS : nothing | ||
| + | |||
| + | =September, 13th= | ||
| + | |||
| + | ==Grow RBS S6== | ||
| + | |||
| + | - Put the 10mL of LB with RBS S6 from yesterday into 200mL of LB with antibiotic | ||
| + | |||
| + | - Incubate at 37°C with shaking | ||
| + | |||
| + | ==RBS W screening== | ||
| + | |||
| + | - Plasmid miniprep of RBS W2, 4, 6, 8, 10 9witouh endo wash buffer) | ||
| + | |||
| + | - Nanodrop | ||
| + | |||
| + | {|class="wikitable" style="text-align:center" border="1" | ||
| + | |- | ||
| + | ! product !! concentration (ng/μL) !! 260/280 !! quantity of DNA to add to have about 300ng (μL) !! added SDW for single digest (μL) | ||
| + | |- | ||
| + | | RBS W2 || 42.4 || 1.62 || 8 || 9 | ||
| + | |- | ||
| + | | RBS W4 || 35.1 || 1.61 || 10 || 7 | ||
| + | |- | ||
| + | | RBS W6 || 41.0 || 1.67 || 8 || 9 | ||
| + | |- | ||
| + | | RBS W8 || 72.8 || 1.61 || 5 || 12 | ||
| + | |- | ||
| + | | RBS W10 || 51.0 || 1.60 || 6 || 11 | ||
| + | |} | ||
| + | |||
| + | - Single digest with EcoRI and XbaI | ||
| + | |||
| + | - Gel1 | ||
| + | |||
| + | *Lane2 : HyperladderI | ||
| + | *Lane3 : RBS W2 cut with EcoRI | ||
| + | *Lane4 : RBS W2 cut with XbaI | ||
| + | *Lane5 : RBS W4 cut with EcoRI | ||
| + | *Lane6 : RBS W4 cut with XbaI | ||
| + | *Lane7 : RBS W5 cut with EcoRI | ||
| + | *Lane8 : RBS W5 cut with XbaI | ||
| + | *Lane9 : RBS W6 cut with EcoRI | ||
| + | *Lane10 : RBS W6 cut with XbaI | ||
| + | *Lane11 : RBS W8 uncut | ||
| + | *Lane12 : supercoiled ladder | ||
| + | |||
| + | |||
| + | - Gel2 | ||
| + | |||
| + | *Lane2 : HyperladderI | ||
| + | *Lane3 : RBS W8 cut with EcoRI | ||
| + | *Lane4 : RBS W8 cut with XbaI | ||
| + | *Lane5 : RBS W10 cut with EcoRI | ||
| + | *Lane6 : RBS W10 cut with XbaI | ||
| + | *Lane7 : RBS W11 cut with EcoRI | ||
| + | *Lane8 : RBS W11 cut with XbaI | ||
| + | *Lane9 : RBS W8 uncut | ||
| + | *Lane10 : supercoiled ladder | ||
| + | |||
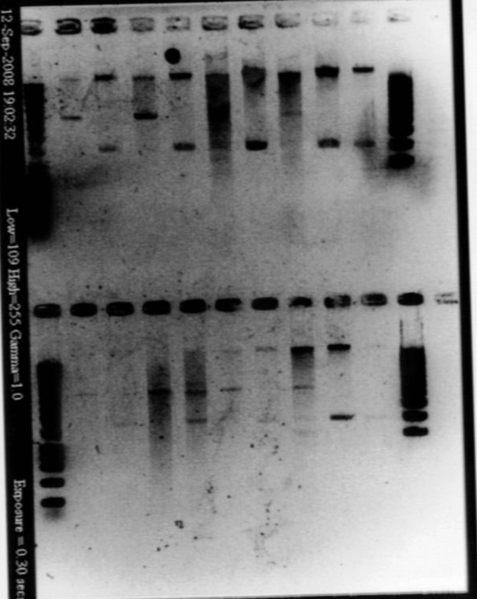
| + | *Result | ||
| + | |||
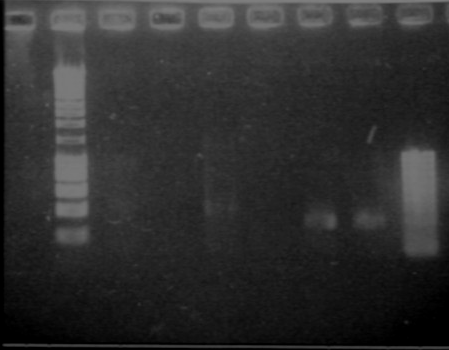
| + | [[Image:gel31091pcr.gif|250px|center]] | ||
| + | |||
| + | - W2 with EcoRI : cut | ||
| + | |||
| + | - W2 with XbaI : uncut, self ligation | ||
| + | |||
| + | - W4 with EcoRI : cut | ||
| + | |||
| + | - W4 with XbaI : uncut, self ligation | ||
| + | |||
| + | - W5 with EcoRI : cut | ||
| + | |||
| + | - W5 with XbaI : uncut, self ligation | ||
| + | |||
| + | - W6 with EcoRI : cut | ||
| + | |||
| + | - W6 with XbaI : cut (2 bands), transformation ok for RBS W6 | ||
| + | |||
| + | - W8 with EcoRI : impossible to see | ||
| + | |||
| + | - W8 with XbaI : impossible to see | ||
| + | |||
| + | - W10 with EcoRI : cut | ||
| + | |||
| + | - W10 with XbaI : maybe cut, it seems to be 2 bands, but quite difficult to see | ||
| + | |||
| + | - W11 with EcoRI : cut | ||
| + | |||
| + | - W11 with XbaI : uncut, self ligation | ||
| + | |||
| + | ==Double check of RBS W6 and stock== | ||
| + | |||
| + | - Grow RBS W6 in 10mL of LB with antibiotic | ||
| + | |||
| + | =September, 14th= | ||
| + | |||
| + | ==RBS S6 and RBS W6== | ||
| + | |||
| + | - Put the 10mL of LB with OBS W6 in 200mL of LB with antibiotic | ||
| + | |||
| + | - Aliquot the 200mL of LB with RBS S6, spin, throw out the supernatant and freeze the pellet | ||
| + | |||
<!-- ## Do not edit below this line unless you know what you are doing. ## --> | <!-- ## Do not edit below this line unless you know what you are doing. ## --> | ||
Revision as of 22:20, 28 October 2008
August, 16th
Agr A and C ligation to pSB4C5
Ligation was performed using Fermentas Rapid Ligation kit according to their protocol.
After ligation, samples were visualized on a gel, but little product of the correct size was seen.
For agrA we should have gotten a band of size 3700 (3000plasmid + 700 insert)
For agrC we should have gotten a band of size 4300 (3000plasmid + 1300insert)
The bands of appropriate sizes were extracted for transformation.
August, 18th
Check promoters
On Friday, PCR out promoters, we want to check them.
- Run a gel
- Results : good size of bands!!!
Transformation of AgrA and AgrC
AgrA and C gell-extracted ligation was transformed into Top 10 cells using standard protocol. Puc9 was used as a positive control.
August, 19th
Results of AgrA and C transformation
Transformation has failed. No colonies were visible for the plates of Agr A or C. The Puc9 positive control grew. We believe that the problem was in the gel-extraction between ligation and transformation. Most of our plasmid was probably lost in this step. Next time we will directly use the results from the ligation reaction to transform. Although many bands were seen on the previous gel of the ligation reaction, we will check our transformation growth by single colony PCR to confirm transformation of the plasmid with our correct insert.
Lux parts
To make the Lux Receiver, we need 4 different parts ;
- R0040, TetR repressible promoter
- SO168, luxR + double terminator
- R0062, promoter activated by luxR
- JO4630 (GFP + double terminator)
All these parts have been transformed into E.coli. We want to test them. R004, R0062 and JO4630 have already been tested, it should be fine. We received from the MIT R0040, R0062 and S068 already transformed into E.coli, so we want to check these stocks (which are certainly fine) and use them. For JO4630, we want to double check our transformation.
- Plate on antibiotic plates and do LB stocks of single colony from the MIT stock (R0040, R0062 and S0168).
- Put on Kan plates 4 different colonies from J04630 (transformation Amp plate) and also incubate these colonies into LB
August, 20th
Check Lux components
- Single colony PCR for :
- R0040 (MIT stuff)
- R0062 (MIT stuff)
- 4 different colonies of S0168 (from a transformation plate from 12/08)
- 4 different colonies of J04630 (from a transformation plate from 12/08)
- Protocol : add 1μL of cells (diluted in water), 10μL of Master Mis, 7μL of SDW, 1μL of VF primer and 1μL of VR primer
- Gel PCR products
Gel 1
- Lane2 : Hyperladder1
- Lane3 : JO4630, colony 1
- Lane4 : JO4630, colony 2
- Lane5 : JO4630, colony 3
- Lane6 : JO4630, colony 4
- Lane7 : HyperladderI
Gel 2
- Lane2 : HyperladderI
- Lane3 : ECE190 double digest
- Lane4 : S0168, colony 1
- Lane5 : S0168, colony 2
- Lane6 : S0168, colony 3
- Lane7 : S0168, colony 4
- Lane8 : HyperladderI
- Lane9 : R0040
- Lane10 : R0062
- Lane11 : Ladder 100bp
- Results
- R0040 and R0062 : one big band of about 300b (expected size 293), OK!
- S0168 : one band of about 400b for the 4 different colonies (expected size 1234!), bad! This plate does not contain S0168
- J04630 (colonies 2 and 4) : one band of about 1100b (expected size 1173), OK!
- J04630 (colony 1) : one good band plus another band...
- J04630 (colony 3) : one band of about 600b, bad!
Ligation
- Materials :
- AgrA
- AgrB
- AgrC
- AgrD
- Pupp
- Pspac
- Ppac
- Pxyl
- RBS S
- RBS W
- psB4C5
- Double digest of PCR products
- Run vector, AgrA and AgrD on a gel
- DNA clean and concentrator for AgrA, B,C and D, promoters
- Microclean for both RBS
- Nanodrop
| 260/280 | ng/μL | |
|---|---|---|
| AgrA | 1.66 | 16.4 |
| AgrB | 1.91 | 23.5 |
| AgrC | 1.99 | 35.9 |
| AgrD | 2.13 | 4.9 |
| Pxyl | 1.54 | 5.6 |
| Ppac | 1.49 | 4.6 |
| Pspc | 1.62 | 9.6 |
| Pupp | 1.88 | 8.5 |
| RBS S | 2.44 | 29 |
| RBS W | 1.44 | 10.7 |
- Extract plasmid annd Agr from gel and clean
- Ligation
August, 21st
Transformation of ligation products
- Spin chemically competent TOP10, add 100μL of CaCl2 solution
- Add 5μL of DNA (ligation products), and 1μL of PUC9
- Continue the protocol of transformation
J04630
- Plate good colonies from yesterday on Kan25 and put in LB (for plasmid stock for tomorrow)
Plasmid stocks
- Plasmid miniprep R0040 and R0062
August, 22nd
New PCR of promoters
August, 23rd
Check promoters (after PCR from bacillus vectors)
- Gel
- Lane1 : Hyperladder 5
- Lane2 : Pxyl
- Lane3 : Pspac
- lane4 : Ppac
- Lane5 : Pupp
Size is ok.
August, 27th
New transformation of ligation products
- Products : agrD, Pupp, Pspac, RBS W, RBS S (from ligation with biolabs kit)
- Competent top 10 from the freezer
- Add 5μL of ligation products, and 1.2μL of PUC9
August, 28th
Results from transformation of ligation products
- nothing on plate, even on control plate
- Reasons ?
- cells non competent anymore (try with fresh competent cells)
- not enough DNA (try with 10μL of DNA)
- very short ligation products
Transformation of ligation products (new)
- Products to ligate : Pupp, Pspac, agrD, RBS S and RBS W
- new fresh competent TOP 10
- 5μL of DNA (1.5μL of PUC9)
- 2h30 in the incubator
August, 29th
Results of transformation with our ligation products
- Everything grew! Better efficiency with electrop. than with chemical protocol
Single colony PCR to check our transformation
| Transformed products | number of picked colonies from chemical transformation | number of picked colonies from electrop. transformation (neat) | number of picked colonies from electrop. transformation (1:10) |
|---|---|---|---|
| Pupp | 2 | 2 | 0 |
| Pspac | 2 | 2 | 0 |
| RBS S | 3 | 0 | 2 |
| RBS W | 3 | 0 | 2 |
| agrD | 3 | 2 | 0 |
- Single colony PCR : 13μL of SDW+cells, 5μL of Master Mix, 1μL of VF and 1μL of VR (and plate each single colony)
- Load a gel (1.3% agarose) : 5μL of PCR products + 1μL of dye (only 1μL of 100b ladder)
In the death plasmid, the VF-VR size is about 280b.
| Transformed products | size of the product (with cutting sites) | expected size after PCR (about) |
|---|---|---|
| Pupp | 255 | 480 |
| Pspac | 125 | 350 |
| RBS S | 56 | 280 |
| RBS W | 56 | 280 |
| agrD | 200 | 430 |
- Result : nothing, even no ladder, problem with the gel!
- run again on a e-gel
- Lane1 :ladder 100bp
- Lane2 : Pupp colony 1
- Lane3 : Pupp colony 3
- Lane4 : Pspac colony 1
- Lane5 : Pspac colony 3
- Lane6 : RBS S colony 1
- Lane7 : RBS S colony 4
- Lane8 : RBS W colony 1
- Lane9 : RBS W colony 4
- Lane10 : agrD colony 1
- Lane11 : agrD colony 4
- Lane12 : HyperladderI
- Result : nothing, just the primers! Problem with our PCR
Plate biobricks from MIT
- E0840
- B0014
- I712007
- C0012
- B0015
- C0061
- R0063
September, 2nd
Check transformation in E.coli of our ligated products
- Single colony PCR with VF and VR : add 10μL of SDW, 5μL of MM, 1μL of VF, 1μL of VR and 3μL of cells
| inserted products | number of colonies to check (chemical transf.) | number of colonies to check (electrop. transf.) |
|---|---|---|
| RBS S | 3 | 2 |
| RBS W | 3 | 2 |
| Pspac | 2 | 2 |
| Pupp | 2 | 2 |
| agrD | 3 | 2 |
- Gel (3% of agarose)
- Result
| gel | lane | inserted product (name of the colony) | observation | conclusion |
|---|---|---|---|---|
| top | 3 | RBS S (1) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 4 | RBS S (2) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 5 | RBS S (4) | nothing | ? |
| top | 6 | RBS S (5) | nothing | ? |
| top | 7 | RBS W (1) | 2 band (260bp + 350bp) | size of the insert, RBS too small to see on a gel + something else? |
| top | 8 | RBS W (2) | one band (350bp) | too big |
| top | 9 | RBS W (4) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 10 | RBS W (5) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 11 | Pspac (1) | 2 band (260bp + 350bp) | maybe problem with products loaded on gel (exactly the same bands than for RBS W) |
| top | 12 | Pspac (2) | one band (450bp) | size of Pupp? |
| top | 13 | Pspac (3) | one band (450bp) | size of Pupp? |
| bottom | 3 | Pspac (4) | nothing | ? |
| bottom | 4 | Pupp (1) | one band (400bp) | size of Pspac? |
| bottom | 5 | Pupp (2) | one band (400bp) | size of Pspac? |
| bottom | 6 | Pupp (3) | one band (slightly lower than 400bp) | size of Pspac? |
| bottom | 7 | Pupp (4) | one band (400bp) | size of Pspac? |
| bottom | 8 | agrD (1) | 2 bands (380 and 450bp) | big band is ok |
| bottom | 9 | agrD (2) | 2 bands (380 and 450bp) | big band is ok |
| bottom | 10 | agrD (3) | no bands | ? |
| bottom | 11 | agrD (4) | strong band (450bp) | ok |
| bottom | 12 | P2 | one band (350bp) | ok |
September, 3rd
Checking the insert of promoters
We ligated promoters into death vector, and transforned into Top 10. Our transformation worked, but it was hard to say with the result of yesterday if we had the good insert. Moreover, it is possible that we inverted Pupp and Pspac. So we are going to make a new single colony PCR with the primers of promoters (we used the same single colonies than yesterday).
We also want to PCR again all promoters (Pspac, Pxyl, Pupp and Ppac) from plasmids to have more.
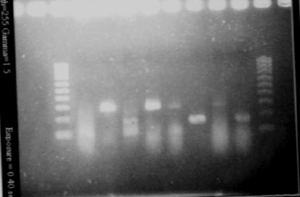
Picture of the gel with the different promoters
| promoter | colony | primers | name |
|---|---|---|---|
| Pspac | 1 | Pspac primers | A |
| Pspac | 1 | Pupp primers | B |
| Pspac | 2 | Pspac primers | C |
| Pspac | 2 | Pupp primers | D |
| Pupp | 1 | Pupp primers | E |
| Pupp | 1 | Pspac primers | F |
| Pupp | 3 | Pupp primers | G |
| Pupp | 3 | Pspac primers | H |
- Single colony PCR : add 10μL of SDW, 5μL of MM, 1μL + 1μL of primers, 3μL of cells
- Gel
- lane 3 : hyperladderI
- lane 4 : Pspac (colony1) with Pspac primers
- lane 5 : Pspac (colony1) with Pupp primers
- lane 6 : Pspac (colony2) with Pspac primers
- lane 7 : Pspac (colony2) with Pupp primers
- lane 8 : Pupp (colony1) with Pupp primers
- lane 9 : Pupp (colony1) with Pspac primers
- lane 10 : Pupp (colony3) with Pupp primers
- lane 11 : Pupp (colony3) with Pspac primers
- lane 12 : HyperladderI
- Results
| promoter | colony | primers | observation | Conclusion |
|---|---|---|---|---|
| Pspac | 1 | Pspac primers | nothing | it is in fact Pupp |
| Pspac | 1 | Pupp primers | band (about 280bp) | Pupp OK |
| Pspac | 2 | Pspac primers | nothing | it is in fact Pupp |
| Pspac | 2 | Pupp primers | band (about 280bp) | Pupp OK |
| Pupp | 1 | Pupp primers | nothing | it is in fact Pspac |
| Pupp | 1 | Pspac primers | band (about 180bp) | Pspac OK |
| Pupp | 3 | Pupp primers | nothing | it is in fact Pspac |
| Pupp | 3 | Pspac primers | band (about 180bp) | Pspac OK |
Make some stock of our new biobricks
- Pupp (colony1) into the death vector, transformed into TOP10
- Pspac (colony1) into the death vector, transformed into TOP10
- agrD (colony5) into the death vector, transformed into TOP10
- Grow this single colony into 10mL of LB (Cm35)
Transformation of new "biobricks"
- transform into E.coli :
- agrA
- agrB
- agrC
- Pxyl
September, 4th
Results of new "biobricks"transformation
- Nothing on our plate, except for the control plate.
New attempt of new "biobricks" transformation
- transform into E.coli these ligated products:
- agrA
- agrB
- agrC
- Pxyl
September, 7th
New PCR
- New stocks of Master Mix
- PCR agrA, agrB, Pxyl, Pspac, Ppac and Pupp
September, 8th
Check product of PCR from yesterday
- Lane3 : Hyperladder I
- Lane4 : agrA
- Lane5 : agrB
- Lane6 : Pxyl
- Lane7 : Pspac
- Lane8 : Ppac
- Lane9 : Pupp
- Lane10 Hyperladder IV
- Results : everything is ok
Check transformation from 03/09
After several days of incubation, we have a few colonies on our transformed plates (agrA, B and C).
- Single colony PCR
- Add 13μL of cells diluted into SDW, 5μL of MM, 1μL of VF and 1μL of VR
- Gel
September, 10th
PCR of GFP+RBS and Promoter+RBS
- PCR
- Run a 1.2% agarose gel with 1μL of sample
- Lane 3 : HyperladderI
- Lane 4 : GFP + RBS 1A
- Lane 5 : GFP + RBS1B
- Lane 6 : GFP + RBS 2A
- Lane 7 : GFP + RBS 2B
- Lane 8 : Pupp + RBS 1
- Lane 9 : Pupp + RBS 2
- Lane 10: HyperladderIV
- Result
RBS screening
- 5 colonies from chemical transformation + 6 colonies from electroporation transformation for RBS S
- 5 colonies from chemical transformation + 6 colonies from electroporation transformation for RBS W
- PCR : 11μL of SDW + 5μL of MM + 1+1μL of primers (RBS detect + VR) + 2μL of cells (program iGEM34)
- Gel1
- Lane2 : RBSS1
- Lane3 : RBS S2
- Lane4 : RBS S3
- Lane5 : RBS S4
- Lane6 : RBS S5
- Lane7 : RBS S6
- Lane8 : HyperladderIV
- Lane9 : RBS S7
- Lane10 : RBS S8
- Lane11 : RBS S9
- Lane12 : RBS S10
- Lane13 : RBS S6 with VF and VR primers
- Lane14 : RBS S11
- Gel2
- Lane2 : RBSW1
- Lane3 : RBS W2
- Lane4 : RBS W3
- Lane5 : RBS W4
- Lane6 : RBS W5
- Lane7 : RBS W6
- Lane8 : HyperladderIV
- Lane9 : RBS W7
- Lane10 : RBS W8
- Lane11 : RBS W9
- Lane12 : RBS W10
- Lane13 : RBS W6 with VF and VR primers
- Lane14 : RBS W11
- Result
- Nothing, except for the PCR with VF and VR primers, either our primers are not right, either we have no insert. But the gel is not good enough to see very well, we will try to run a new gel.
September, 11th
RBS screening
- We run our PCR products from yesterday on a new 1.8% agarose gel
- Gel1
- Lane2 : RBSS1
- Lane3 : RBS S2
- Lane4 : RBS S3
- Lane5 : RBS S4
- Lane6 : RBS S5
- Lane7 : RBS S6
- Lane8 : HyperladderIV
- Lane9 : RBS S7
- Lane10 : RBS S8
- Lane11 : RBS S9
- Lane12 : RBS S10
- Lane13 : RBS S6 with VF and VR primers
- Lane14 : RBS S11
- Gel2
- Lane2 : RBSW1
- Lane3 : RBS W2
- Lane4 : RBS W3
- Lane5 : RBS W4
- Lane6 : RBS W5
- Lane7 : RBS W6
- Lane8 : HyperladderIV
- Lane9 : RBS W7
- Lane10 : RBS W8
- Lane11 : RBS W9
- Lane12 : RBS W10
- Lane13 : RBS W6 with VF and VR primers
- Lane14 : RBS W11
- Result
- Nothing, except for the PCR with VF and VR primers, either our primers are not right, either we have no insert.
RBS screning but single digest
- Grow some colonies of Top10 transformed with RBS into 10mL of LB with antibiotic
We want to check our transformation with single digest. If we have self ligation in our transformation, we will loose the XbaI cutting site.
Backbone for ligation
- New pellets in 600μL of SDW
- Plasmid miniprep
September, 12th
RBS screening (single digest)
- Plasmid miniprep 6 colonies of RBS S and 6 colonies of RBS W (without endo wash buffer)
- Nanodrop
| product | concentration (ng/μL) | 260/280 | quantity of DNA to add to have about 300ng (μL) | added SDW for single digest (μL) |
|---|---|---|---|---|
| RBS W1 | 57.1 | 1.57 | 6 | 11 |
| RBS W3 | 54.1 | 1.53 | 6 | 11 |
| RBS W5 | 15.1 | 1.85 | 17 | 0 |
| RBS W 7 | 21.8 | 1.66 | 15 | 2 |
| RBS W9 | 21.8 | 1.78 | 15 | 2 |
| RBS W11 | 13.8 | 1.73 | 17 | 0 |
| RBS S2 | 19.5 | 1.7 | 15 | 2 |
| RBS S4 | 82.13 | 2.57 | 4 | 13 |
| RBS S6 | 44.7 | 1.64 | 7 | 10 |
| RBS S8 | 104.0 | 1.48 | 3 | 14 |
| RBS S10 | 13.1 | 1.82 | 17 | 0 |
| RBS S11 | 20.1 | 1.73 | 15 | 2 |
- Single digest : with EcoRI and XbaI, add SDW and DNA (according to the previous table, 300mg of DNA), 2μL of fast digest buffer, and 1μL of enzyme
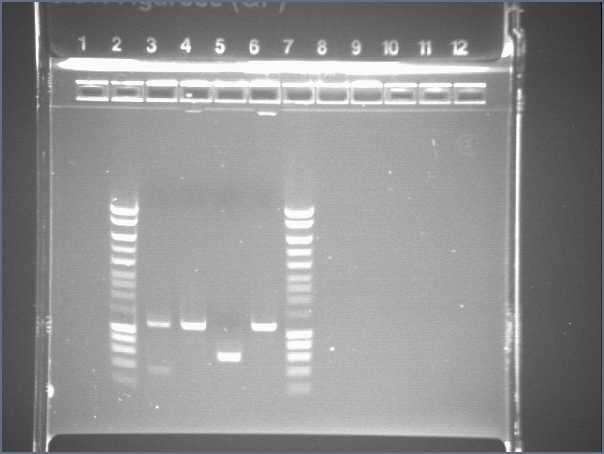
- Gel1
- Lane2 : HyperladderI
- Lane3 : RBS W1 cut with EcoRI
- Lane4 : RBS W1 cut with XbaI
- Lane5 : RBS W3 cut with EcoRI
- Lane6 : RBS W3 cut with XbaI
- Lane7 : RBS W7 cut with EcoRI
- Lane8 : RBS W7 cut with XbaI
- Lane9 : RBS W9 cut with EcoRI
- Lane10 : RBS W9 cut with XbaI
- Lane11 : RBS W9 uncut
- Lane12 : supercoiled ladder
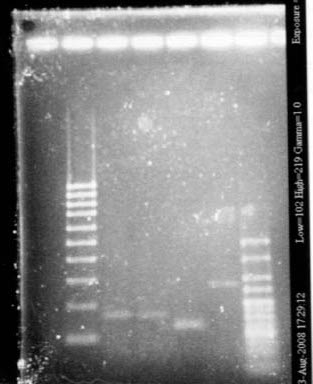
- Gel2
- Lane2 : HyperladderI
- Lane3 : RBS S4 cut with EcoRI
- Lane4 : RBS S4 cut with XbaI
- Lane5 : RBS S6 cut with EcoRI
- Lane6 : RBS S6 cut with XbaI
- Lane7 : RBS S8 cut with EcoRI
- Lane8 : RBS S8 cut with XbaI
- Lane9 : RBS S11 cut with EcoRI
- Lane10 : RBS S11 cut with XbaI
- Lane11 : RBS S4 uncut
- Lane12 : supercoiled ladder
- Result
The size of our plasmid is about 3000bp (ok on the gel)
- RBS W
- W1 with EcoRI : cut
- W1 with XbaI : uncut, self ligation
- W3 with EcoRI : cut
- W3 with XbaI : uncut, self ligation
- W7 with EcoRI : impossible to see
- W7 with XbaI : uncut, self ligation
- W9 with EcoRI : cut
- W9 with XbaI : uncut, self ligation
- RBS S
- S4 with EcoRI : cut
- S4 with XbaI : uncut, self ligation
- S6 with EcoRI : cut
- S6 with XbaI : 2 band, this plasmid is partially cut!!! Transformation with RBS S
- S8 with EcoRI : cut
- S8 with XbaI : uncut, self ligation
- S11 with EcoRI : cut
- S11 with XbaI : uncut, self ligation
Double check of RBS S6 and stock
- Grow RBS S6 in LB with Cm35
Check more RBS W
- Grow RBS W2, 4, 6, 8, 10 in 10mL of LB with antibiotic
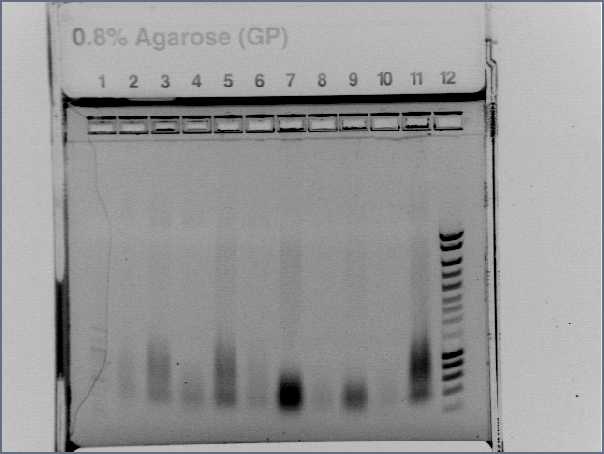
Check PCR products
- Gel (2μL of each product)
- Lane1 : λ ladder
- Lane2 : agrA
- Lane3 : agrB
- Lane4 : agrC
- Lane5 : rep
- Lane6 : pSB4C5 1
- Lane7 : pSB4C5 2
- Lane8 : Pxyl
- Lane9 : Ppac
- Lane10 : RBS S
- Lane11 : RBS W
- Lane12 : HyperladderI
- Results
- agr B : 2 bands?
- agrC : ok
- rep : small band but good size
- backbones : ok
- promoters : ok
- RBS : nothing
September, 13th
Grow RBS S6
- Put the 10mL of LB with RBS S6 from yesterday into 200mL of LB with antibiotic
- Incubate at 37°C with shaking
RBS W screening
- Plasmid miniprep of RBS W2, 4, 6, 8, 10 9witouh endo wash buffer)
- Nanodrop
| product | concentration (ng/μL) | 260/280 | quantity of DNA to add to have about 300ng (μL) | added SDW for single digest (μL) |
|---|---|---|---|---|
| RBS W2 | 42.4 | 1.62 | 8 | 9 |
| RBS W4 | 35.1 | 1.61 | 10 | 7 |
| RBS W6 | 41.0 | 1.67 | 8 | 9 |
| RBS W8 | 72.8 | 1.61 | 5 | 12 |
| RBS W10 | 51.0 | 1.60 | 6 | 11 |
- Single digest with EcoRI and XbaI
- Gel1
- Lane2 : HyperladderI
- Lane3 : RBS W2 cut with EcoRI
- Lane4 : RBS W2 cut with XbaI
- Lane5 : RBS W4 cut with EcoRI
- Lane6 : RBS W4 cut with XbaI
- Lane7 : RBS W5 cut with EcoRI
- Lane8 : RBS W5 cut with XbaI
- Lane9 : RBS W6 cut with EcoRI
- Lane10 : RBS W6 cut with XbaI
- Lane11 : RBS W8 uncut
- Lane12 : supercoiled ladder
- Gel2
- Lane2 : HyperladderI
- Lane3 : RBS W8 cut with EcoRI
- Lane4 : RBS W8 cut with XbaI
- Lane5 : RBS W10 cut with EcoRI
- Lane6 : RBS W10 cut with XbaI
- Lane7 : RBS W11 cut with EcoRI
- Lane8 : RBS W11 cut with XbaI
- Lane9 : RBS W8 uncut
- Lane10 : supercoiled ladder
- Result
- W2 with EcoRI : cut
- W2 with XbaI : uncut, self ligation
- W4 with EcoRI : cut
- W4 with XbaI : uncut, self ligation
- W5 with EcoRI : cut
- W5 with XbaI : uncut, self ligation
- W6 with EcoRI : cut
- W6 with XbaI : cut (2 bands), transformation ok for RBS W6
- W8 with EcoRI : impossible to see
- W8 with XbaI : impossible to see
- W10 with EcoRI : cut
- W10 with XbaI : maybe cut, it seems to be 2 bands, but quite difficult to see
- W11 with EcoRI : cut
- W11 with XbaI : uncut, self ligation
Double check of RBS W6 and stock
- Grow RBS W6 in 10mL of LB with antibiotic
September, 14th
RBS S6 and RBS W6
- Put the 10mL of LB with OBS W6 in 200mL of LB with antibiotic
- Aliquot the 200mL of LB with RBS S6, spin, throw out the supernatant and freeze the pellet
 "
"