Team:Tokyo Tech/Protocol
From 2008.igem.org
(Difference between revisions)
(→Reporter Assay) |
(→Reporter Assay) |
||
| Line 20: | Line 20: | ||
# Overnight cultures of reporter strains grown at 37℃ in LB medium containing appropriate antibiotics were diluted 1:100 in LB medium | # Overnight cultures of reporter strains grown at 37℃ in LB medium containing appropriate antibiotics were diluted 1:100 in LB medium | ||
# Incubate at 37℃ as fresh cultures | # Incubate at 37℃ as fresh cultures | ||
| - | # After their OD<sub>600</sub> | + | # After their OD<sub>600</sub> reach 0.6, dilute the fresh cultures 1:1 in various concentrations of IPTG [0μM, 1 μM, 10 μM, 20 μM, 50μM, 100 μM and 1000 μM]. |
# The fresh cultures were incubated at 37℃ for 12 hours | # The fresh cultures were incubated at 37℃ for 12 hours | ||
| - | # Every 2 hours, 200 μl of each culture | + | # Every 2 hours, take 200 μl of each culture to 1.5 ml tube and '''wash''' it. |
| - | + | ||
| - | + | ||
# Take 150 μl of the washed culture to 96-well plate and measure its fluorescence intensity by fluorimeter (FLA). | # Take 150 μl of the washed culture to 96-well plate and measure its fluorescence intensity by fluorimeter (FLA). | ||
==== wash ==== | ==== wash ==== | ||
| - | + | # Centrifuge for 2 min at 9000g | |
| + | # Discared the spernatant with a pipette | ||
| + | # Dissolve the pellet at the bottom of the tube in 200 μl of PBS | ||
| + | # Centrifuge for 2 min at 9000g | ||
| + | # Discared the spernatant with a pipette | ||
| + | # Dissolve the pellet at the bottom of the tube in 200 μl of PBS | ||
| + | |||
| - | The measured fluorescence intensity was corrected by subtracting the background fluorescence, measured in control wells containing 150 μl of PBS. The corrected value was normalized to culture volume and | + | The measured fluorescence intensity was corrected by subtracting the background fluorescence, measured in control wells containing 150 μl of PBS. The corrected value was normalized to culture volume and OD<sub>600</sub> and expressed in fluorescence per (ml x OD<sub>600</sub>). A strain, transformed with PtetR-GFP, which expresses GFP constitutively, was used as a positive control. A strain, transformed with Promoter less-GFP which does not express GFP was used as a negative control. |
Revision as of 22:51, 29 October 2008
| Main | Protcol | Parts Submitted to the Registry | Our Team | Acknowledgements |
|---|
Protocol
Reporter Assay
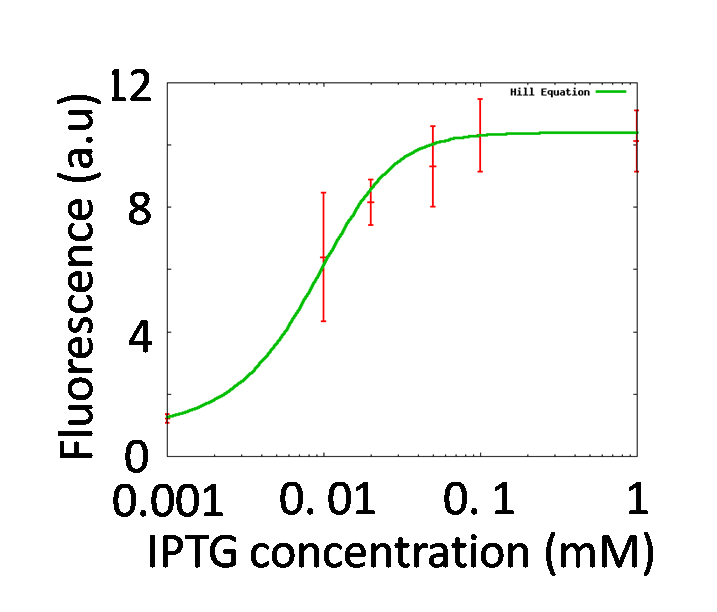
To quantitatively determine the performances of operator parts in the reporter plasmid, the change of fluorescence intensities of the reporter strains in the various concentrations of IPTG were measured.
- Overnight cultures of reporter strains grown at 37℃ in LB medium containing appropriate antibiotics were diluted 1:100 in LB medium
- Incubate at 37℃ as fresh cultures
- After their OD600 reach 0.6, dilute the fresh cultures 1:1 in various concentrations of IPTG [0μM, 1 μM, 10 μM, 20 μM, 50μM, 100 μM and 1000 μM].
- The fresh cultures were incubated at 37℃ for 12 hours
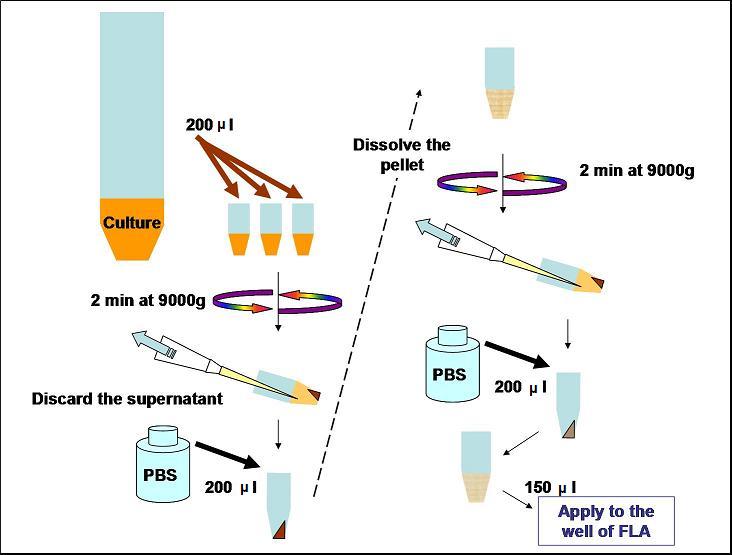
- Every 2 hours, take 200 μl of each culture to 1.5 ml tube and wash it.
- Take 150 μl of the washed culture to 96-well plate and measure its fluorescence intensity by fluorimeter (FLA).
wash
- Centrifuge for 2 min at 9000g
- Discared the spernatant with a pipette
- Dissolve the pellet at the bottom of the tube in 200 μl of PBS
- Centrifuge for 2 min at 9000g
- Discared the spernatant with a pipette
- Dissolve the pellet at the bottom of the tube in 200 μl of PBS
The measured fluorescence intensity was corrected by subtracting the background fluorescence, measured in control wells containing 150 μl of PBS. The corrected value was normalized to culture volume and OD600 and expressed in fluorescence per (ml x OD600). A strain, transformed with PtetR-GFP, which expresses GFP constitutively, was used as a positive control. A strain, transformed with Promoter less-GFP which does not express GFP was used as a negative control.
 "
"