Team:University of Lethbridge/Notebook/Project1July
From 2008.igem.org
Contents |
July 1, 2008
Nathan Puhl
Subcultured RP1616 into liquid LB and pTopp cells into liquid LB + amp
July 2, 2008
Nathan Puhl, Christa, Munima
Plasmid mini prepped pTopp cells.
Attempted to make RP1616 cells competent using the open wet ware [http://openwetware.org/wiki/Preparing_chemically_competent_cells protocol]. 100 uL aliquots of cells were frozen in liquid nitrogen and stored in the -80 freezer.
Ran CheZ in pUC19 plasmid and pUC19 plasmid from last year with high range ladder. The pUC19 plasmid seems weird, I don't think it should have two bands; I will have to ask someone about that. The CheZ plasmid appears to be the right size (2600 + 620 = 3220 bp)
July 3, 2008
Nathan Puhl, Munima, Christa
No colonies on either plate, but the max-efficiency DH5-alpha cells transformed with pSB1A7 were successfully transformed (~1500 cfu). There are two possibilities: 1. the cells are competent but are low efficiency and we did not add enough DNA, or 2. The cells are not competent. To assess possibility one we will try more DNA. If that does not work we will try a protocol using electroporation.
July 8, 2008
Munima, Christa
Objective: Attempt to transform pSB1A7 into RP1616 cells (made competent on July 2/08) again, but using more DNA and changes to June 16/08 transformation protocol
1. Thawed RP1616 cells and pSB1A7 on ice (obtained from Wieden -80 C freezer) 2. Added 50uL of RP1616 cells to a 15mL falcon tube (prechilled) 3. Left on ice for 10 minutes 4. Added 4uL of pSB1A7 to falcon tube 5. Incubated on ice for 30 minutes 6. Heat shocked without shaking for 45 seconds at 42 C 7. Placed on ice for 2 minutes 8. Added 1mL of SOC media preheated to 42 C. Incubated for 1 hour at 37 C in shaker incubator 9. Put a 400uL aliquot of mixture from incubator into a centrifuge tube 10. Centrifuged for 1 minute on max speed 11. Poured off supernatant and resuspended in 100uL of SOC media 12. Plated 100uL on LB + amp plate. Left overnight in incubator.
July 9, 2008
Munima, Christa
Growth observed on amp plate that was plated with transformed competent RP1616 cells with pSB1A7. Approximately 10 colonies were observed, but they look strange.
Subcultured one colony into 5mL LB + amp culture tube (50ug/mL ampicillin). Left overnight in shaker incubator at 37 C at 225 RPM.
July 10, 2008
Munima, Christa
Did plasmid mini prep on RP1616 competent cells with pSB1A7 from the culture tube left overnight. Eppendorf FastPlasmid mini kit was used and the tube was stored in the -20 C iGEM freezer labelled "comp RP1616 + pSB1A7".
July 15, 2008
Munima, Christa
Objective: Make RP1616 cells competent via electroporation
Inoculated two 5 mL culture tubes with RP1616. Left overnight in shaker incubator at 37 C. Inoculations will be used for electroporation on July 16, 2008
July 16, 2008
Christa
Objective for today: Attempt to use electroporation to transform pTopp or pSB1A7 into RP1616 cells.
Made a 1/5 dilution of cells in culture tubes. Left dilution in shaker incubator at 37 C (300RPM) for approximately 4 hours.
Christa, Munima, Selina
Machine: Eppendorf Electroporator 2510
Plasmids pSB1A7 and pTopp will be used.
1. Spun down 2.0 mL of cell culture in two transfers (first added 1.5 mL, removed supernatant, then added another 0.5 mL of culture) into 1.5 mL centrifuge tubes. (Four centrifuge tubes in total) 2. Poured off supernatant. Resuspended in 1 mL of ice cold water to wash (2x). 3. Resuspended in 100 uL of 10% glycerol in water. 4. Added 2 uL of plasmid. Sat for 10 min on ice. (Two tubes for each plasmid) 5. Poured mixture into prechilled 0.1 cm cuvette. 6. Electroporated with 1.5 kV for 2.4 msec. 7. Immediately added 1 mL of media. Three of four tubes were spun down and resuspended. All were in SOC media at completion. 8. Transferred to 15 mL Falcon tube. 9. Left on shaker incubator at 300 RPM and 37 C for approx. 1.5 hours
Munima
10. Took 400 uL aliquots of culture and centrifuged at max for 1 min. 11. Poured off supernatant and resuspended in 100 uL of SOC media. 12. Plated 100 uL on LB + Amp plates. 13. Left to incubate overnight at 37 C.
July 17, 2008
Selina
Checked LB + Amp plates of electroporated RP1616 cells 16 hours later (next morning). Plates showed thick growth (no isolated colonies) in localized swirly patterns
- yesterday cell-media culture was plated by swirling plate on benchtop and growth possibly reflects that - if so, then very likely the electroporation/transformation was sucessful :) - possible problem of satellite colonies? amp may have been used up with such thick growth... - wrapped plates in parafilm and placed in 4 C fridge for storage - future plans: use plate to inoculate culture tubes with amp and attempt to replate for isolates (once more plates are poured...
Plated other half (less than 500 uL) of cell-SOC media culture on LB + Amp plates.
- Cultures were sitting at RT overnight and likely have entered death phase... - Took cell cultures, transferred to microcentrifuge tubes, spun and resuspended in 100 uL LB. - Spread-plated cell cultures (4 total, 2 per attempted plasmid transformation) on LB + Amp plates. - Left to incubate (at ~8:15 am) at 37 C. - note for future attempts: if this comes up again, then try replenishing liquid media in old cell culture - try to revive/awaken any dormant cells..? ask an actual microbiologist what would be smarter to do
Christa
Streaked colonies of pSB1A7 and pTopp onto Brent's LB + amp plates because there was too much growth on the plates observed today.
July 18, 2008
Sebastian, Munima, Christa, Selina
Observed very small colonies on streak plates (LB + amp) of pSB1A7 and pTopp (RP1616).
July 19, 2008
Christa
Objective: Isolate plasmids pSB1A7 and pTopp from electroporated RP1616 (transformed July 16)
Procedure:
1. Picked one isolated colony from RP1616 and plasmids pSB1A7/pTopp (from LB+amp plate from July 17) to inoculate 5 mL of LB liquid media. 2. Repeated with 2nd colony for both plasmid transformations. (Total of 4 inoculated culture tubes). 3. Placed all 4 tubes in shaker incubator at 225 RPM at 37 C for 3 hrs.
Selina, Christa
Objective: Create glycerol stock of potentially transformed RP1616 cells (performed in conjunction with plasmid prep/check)
Procedure:
1. Took absorbance readings at 600 nm of cell cultures after 3 hours (aiming for an optimal reading of 0.6 OD)
-RP1616 + pSB1A7 = 0.561 = good! (only checked one of two culture tubes)
-RP1616 + pTopp (#1) = 0.082 = very low
-RP1616 + pTopp (#2) = 0.051 = very low
2. Ignored RP1616 + pTopp cultures - no growth observed... discarded culture tubes.
3. Created four glycerol stocks (two per culture tube) of (hopefully) transformed RP1616 + pSB1A7 using two methods:
-1st method (one tube): added 750 uL of cell culture to 750 uL of 40% glycerol into 1.5 mL microfuge tube,
vortexed and stored in Wieden -80 C freezer
-2nd method (three tubes): added 0.5 mL cell culture to pre-aliquotted 0.5 glycerol tubes (likely 40%
glycerol... but need to double-check with people with HJ's lab) into 1 mL cryogenic tube. Stored in HJ's -80 C
freezer.
Parafilmed and moved streak plates of RP1616 + pSB1A7/pTopp to 4 C fridge.
Sebastian, Munima
Procedure:
1. Performed a plasmid miniprep of electroporated (July 16) RP1616 + pSB1A7 cells using Eppendorf FastPlasmid kit
(one per culture tube, two total)
- Minor modification: used 2.25 mL cell culture per tube instead of 1.5 mL.
2. Stored isolated plasmids in iGEM -20 C freezer, labelled as "pSB1A7 (RP1616) July 19/08"
July 21, 2008
Christa, Munima, Selina
Objective: To isolate pTopp from electroporated RP1616 + pTopp cells
- Reinoculated two tubes of 5 mL LB + Amp with electroporated cells (RP1616 + pTopp) from LB + Amp plate (July 16/08) - Placed in 37 C shaking incubator overnight (225 RPM)
July 22, 2008
Christa, Munima
Objective: To isolate pTopp from electroporated RP1616 + pTopp cells
1. Performed a plasmid miniprep of electroporated (July 16) RP1616 + pTopp cells using Eppendorf FastPlasmid kit (one per culture tube, two total) - minor modification: used 2.25 mL cell culture per tube instead of 1.5mL 2. Stored isolated plasmids in iGEM -20 C freezer, labelled as "pTopp (RP1616) July 22/08 3. Made 4 glycerol stocks of electroporated RP1616 + pTopp. Stored in -80 C labelled as RP1616 pTopp glycerol stock July 22/08
Selina, Munima
Objective: To determine whether the pSB1A7 transformation and plasmid prep were successful
1. Found concentration of 1:10 dilution of DNA using Brent's ultraspec (all A260/A280 calculations preset into the machine) to be 8.5 ng/uL. 2. Set up EcoRI digest of plasmid using: -2 uL plasmid + 2 uL EcoRI (Invitrogen) enzyme (10 U/uL), total of 20 uL -Digest sat for 4 hours at 37 C -note: intended 1 hour incubation with 1 uL enzyme
Selina, Christa
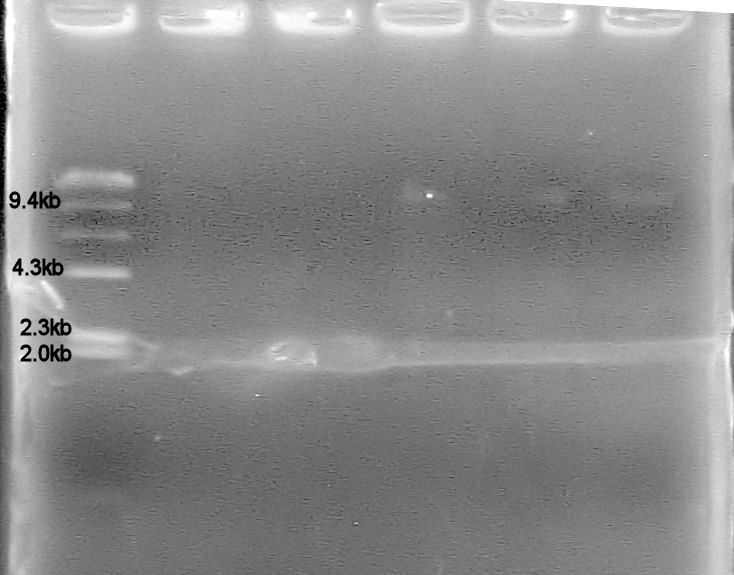
3. Ran 1% TAE agarose gel at 100V -Set up lanes with: 1 uL Lambda-HindIII ladder, 2 uL plasmid, 2 uL EcoRI digest of plasmid, 2 uL plasmid, 1 uL kb ladder -Ran gel too far? Only 1 kb ladder still present
July 24, 2008
Munima, Christa, Selina
Objective: Visualize pSB1A7 and pTopp plasmids extracted from electroporated RP1616 cells. (note: general lab PCRs also run alongside in this gel)
Ran 1% TAE agarose gel at 100V
-Set up lanes with: 5 uL Lambda-HindIII ladder, 5 uL pSB1A7 plasmid, 5 uL EcoRI digest of pSB1A7 plasmid, 7.5 uL pTopp plasmid, 7.5 uL of LacI, 7.5 uL of DT -Plasmid pSB1A7 size = 2.431 kb, any bands...? Possibly a faint band at 10.5 kb? (way too large!?) -Plasmid pTopp size = ___ kb, band size ~ 10 kb
July 27, 2008
Munima, Selina
Objective: Amplify CheZ gene from pTopp plasmid (prepped from E. coli DH5a stock cells, July 2) to find working PCR conditions (positive control for future PCRs)
PCR reaction setup:
-Enzyme = Phusion -Template = pTopp plasmid, dilution series of 1:10, 1:100, 1:1000 and (-) control -Buffer = HF Phusion buffer -Primers = IDT, designed & shipped July 16/08 -Total # of reactions = 20
PCR cycle conditions (programmed into Ute's PCR machine under "iGEMCheZgrad":
1. Initial denaturation @ 98C for 4 mins (1 cycle)
2a. Denaturation @ 98 C for 30 sec (35 cycles for step #2)
2b. Annealing via gradient (5 different annealing temps tested)
-41.0 C
-45.5 C
-49.1 C
-53.8 C
-55.0 C
2c. Extension @ 72C for 30 sec
3. Final extension @ 72C for 7 mins (1 cycle)
4. Hold at 4C
Note: this is the 2nd attempt at amplifying CheZ. Attempt #1 was combined with PCRs for the general lab section... see that webpage for details. Reaction setup was identical, but cycling conditions (including annealing temperature) were adjusted for round #2.
July 28, 2008
Christa, Munima, Selina
Objective: Retry plasmid prep of pSB1A7 (RP1616) and pTopp(RP1616) using Brent's Qia miniprep kit
Today: Streaked glycerol stocks of pSB1A7 (RP1616) (stocked July 19/08) and pTopp (RP1616) (stocked July 22/08) onto LB + Amp plates. Left to incubate overnight at 37 C.
Roxanne, Selina
Objective: Visualize whether any CheZ PCR reactions (July 27) were successful.
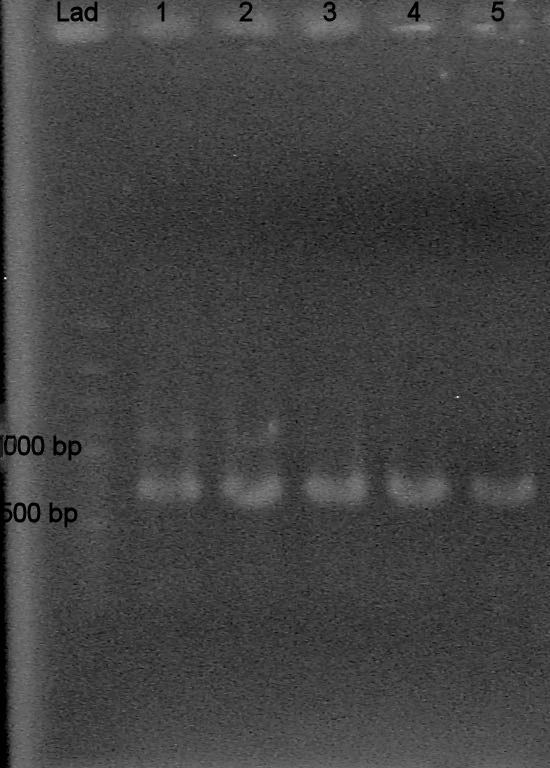
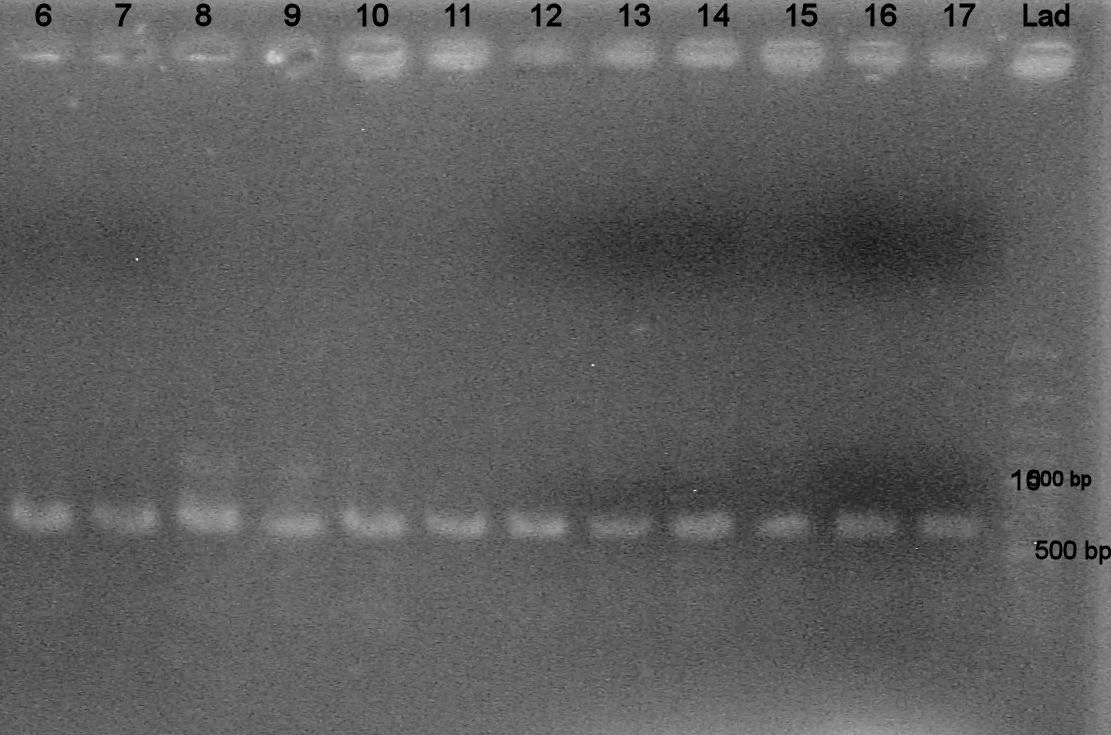
Ran 1% TAE agarose gel at 100 V for 30 mins
Samples:
-Lanes "lad": 100 bp ladder -Lanes 1-5: 1:10 template dilution, annealing temps (41.0, 45.5 C, 49.1 C, 53.8 C, 55.0 C) -Lanes 6-10: 1:100 dil, ann. temp (41.0, 45.5 C, 49.1 C, 53.8 C, 55.0 C) -Lanes 11-15: 1:1000 dil, ann. temp (41.0, 45.5 C, 49.1 C, 53.8 C, 55.0 C)
Results? Bands present in all lanes! (all around 300 bp?)
note: didn't have room on gel to run negative controls - need to do that soon...
 "
"