|
← Yesterday ↓ Calendar ↑Tomorrow →
Amplification of Promotors of interest (FliA, FliL, FlgA, FlgB, FlhB, FlhDC)
We performed PCR on to amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.
PCR Protocol
- Preparation of the templates :--> Resuspend of 1 colony of MG1655 in 100µl of water.
| Number
| Name
| Sequence
| Length
| Comments
|
| O100
| FlgA-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGAGCATATCTCCTCCGCAGGTATCAAAAT
| 58
|
|
| O101
| FlgA-R
| GTTTCTTCCTGCAGCGGCCGCTACTAGTAACAGTATCGCGATGATCGCCACGCTACGT
| 58
|
|
| O102
| FlgB-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGACAGTATCGCGATGATCGCCACGCTACG
| 58
|
|
| O103
| FlgB-R
| GTTTCTTCCTGCAGCGGCCGCTACTAGTAAGCATATCTCCTCCGCAGGTATCAAAATT
| 58
|
|
| O108
| FlhB-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGCCACGTCATATCAGGCGGTCTGATAAGG
| 58
|
|
| O109
| FlhB-R
| GTTTCTTCCTGCAGCGGCCGCTACTAGTAGTTTTGTCGTCGCTCTCGTCAGACACGTC
| 58
|
|
| O111
| FlhDC-Total-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGTCATTTTTGCTTGCTAGCGTACGGAAAA
| 58
| Amplify the both OmpR binding site
|
| O113
| FlhDC(nu)-R
| GTTTCTTCCTGCAGCGGCCGCTACTAGTACAGAATAACCAACTTTATTTTTATG
| 54
| Don't amplify the natural rbs of FlhD (only promoter)
|
| O124
| FliL-F
| GTTTCTTCGAATTCGCGGCCGCTTCTAGAGCAGCGAGAGGCTGTTGGTATTAATGACT
| 58
|
|
| O125
| FliL-R
| GTTTCTTCCTGCAGCGGCCGCTACTAGTACCAGCGATGAAATACTTGCCATGCGATTT
| 58
|
|
For each samples,
1 µl dNTP
10 µl Buffer Phusion 5x
2,5 µl Oligo_F
2,5 µl Oligo_R
1µl template
1 µl Phusion
50 µl qsp H2O (33µl)
- Program PCR: Annealing 55°C - Time élongation 1'30" - Number cycle : 29
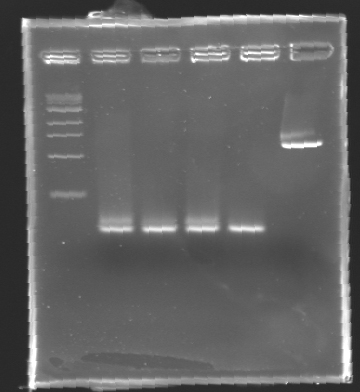
Electrophoresis Purification of PCR
When the PCR cycles were finished,
- 10 µL of 6X loading dye were added
- The samples were then loaded (2 x 30 µL per sample) on a 1,5% agarose gel.
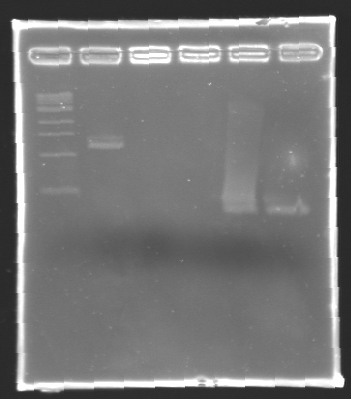
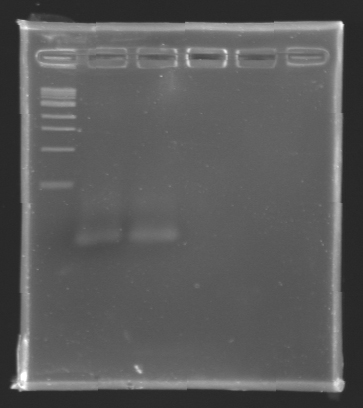
After electrophoresis, the bands corresponding to the right amplification were excised and purified using the QIAquick DNA Gel Extraction Kit (QIAGEN). The elution was made in 50 µL of water. Because the intensity of the band corresponding to MP 120 was very low, we only continued with MP 100. MP 100 was digested by EcoRI & SpeI (Forward Insert) or by XbaI & PstI (D100 : Backward Insert).
| Name
| Promotor
| Gel
| Band
| Expected size
| Measured size
|
| PCR_124
| pFlgA
|
|
|
|
|
| PCR_125
| pFlgB
|
|
|
|
|
| PCR_126
| pFlhB
|
|
|
|
|
| PCR_127
| pFlhDC
|
|
|
|
|
==> Remark :
DNA Digestion
Digestion reaction (total volume : 50 µL)
- 25 µL DNA
- 5 µL buffer 2 (10X)
- 2 µL enzyme 1
- 2 µL enzyme 2
- 0.5 µL BSA (100X)
- 15,5 µL water
- Incubate 2 hours at 37°C
- The samples (2 x 30 µL per sample) were then analysed by electrophoresis.
Electrophoresis (bis)
The sequence of MP 100 (B0034) digested by EcoRI & SpeI (35 bp) or XbaI & PstI (34 bp) was too short and we didn't manage to visualise it on the gel.
Conclusion : small parts like B0034 can't be cloned as an insert.
|
 "
"