|
← Yesterday ↓ Calendar ↑Tomorrow →
Screening of the cloning of OmpR*, EnvZ* and FlhDC+promotor
Electrophoresis
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
| 10
| 11
| 12
| 13
|
| sample
| 1 kb DNA ladder
| positive control:
pSB3K3 (strain S158)
| negative control:
no template
| OmpR*
| EnvZ*
| FlhDC+promotor
| 1 kb DNA ladder
|
| ligation/clone
|
|
|
| L133.1
| L133.2
| L133.3
| L134.1
| L134.2
| L134.3
| L132.1
| L132.2
| L132.3
|
|
| expected size
|
| 316 bp
| 0 kb
| about 1 kb
| about 1,5 kb
| 1210 bp
|
|
| measured size
|
| 1 kb
| 0 kb
| 1 kb
| 0 kb
| 0 kb
| 0 kb
| 1,4 kb
| <0,5 kb
| 1 kb
| 1 kb
| 1 kb
|
|
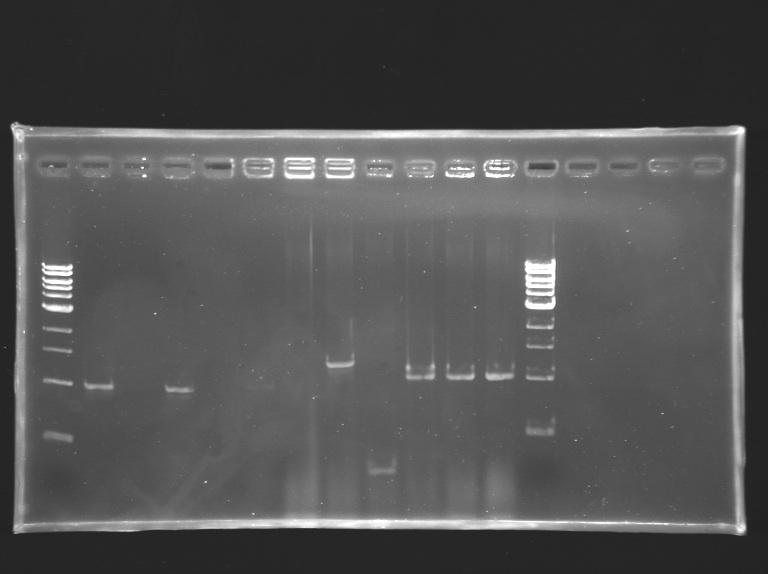 Screening of the cloning of OmpR*, EnvZ* and FlhDC+promotor Minipreps and glycerol stock
- The clones L133.2 and L133.3 didn't grow up in LB+ampicillin: they seem not to have the plasmid, as revealed by PCR.
- Minipreps and Glycerol stocks were made for the clones L133.1 and L134.2.
| Miniprep
| Glycerol Stock
| Ligation
| Name
|
| MP155
| S154
| L133.1
| OmpR*
|
| MP156
| S155
| L134.2
| EnvZ*
|
- Minipreps of L133.1 and L134.2 will be sequenced.
Screening of the cloning of E0240 and FlhDC+promotor
Spreading the clones in order to obtain single colonies
| Strain
| Resistance
| Ligation
| DNA cloned
| vector
| expected size of the fragment amplified by VF & VR
| mesured size
|
| S159.1
| kanamycine
| L139.1
| E0240 (GFP tripart)
| pSB3K3
| 1192 bp
| 1,5 kb
1,1 kb
0,6 kb
|
| S161.1
| ampicilline
| L142.7
| FlhDC+promotor
| pSB1A2
| 1403 bp
| 1,4
0,4 kb
0,3 kb
|
The PCR screening of the transformants L139 and L142 of august 15th revealed several bands for a given clone including one band appearing at the right size.
There are 2 hypothesis:
- The right clone was contaminated by a wrong one
- The clone contains 2 plasmids: one with the insert and another one without the insert
In order to check these 2 hypothesis and to isolate (if it is possible) the right clone (containing the plasmid with the insert). We decided to spread the "clone" in question in a LB plate in order to carry out a PCR screening on single colonies.
- Take of some bacteria from the glycerol stock
- Resuspension in 400 µL of LB+antibiotic
- Spreading of 200 µL in a LB agar plate containing the appropriate antibiotic
- Incubation overnight at 37°C
Promoter characterization plasmids
Ligation
| Ligation name
| Vector digestion
| Vector description
| Vector volume
| Insert digestion
| Insert description
| Insert volume
| Product description
|
| L155
| D164
| J23101 promoter
| 10
| D163
| gfp generator
| 2
| J23101 promoter-gfp generator
|
| L156
| D161
| pTet promoter
| 1
| D163
| gfp generator
| 4
| pTet promoter-gfp generator
|
|
| D161
| 1
|
|
|
|
| Vector autoligation control
|
| L157
| D125.2
| B0015
| 3
| D162
| 4
| tetR
| tetR-B0015
|
|
| D125.2
| 3
|
|
|
|
| Vector autoligation control
|
Digestion
Measurement of concentration of minipreps
to be modified
standard protocol
| Plasmid
| Miniprep
| Concentration (µg/mL)
| ratio 260/280
|
| MP3
| 3
|
|
|
| MP3
| 4
|
|
|
| MP119
| 1
|
|
|
| MP119
| 2
|
|
|
| MP119
| 3
|
|
|
Digestion
to be modified
Protocol Digestion
| Plasmid
| Description
| Miniprep used
| Enzymes
|
| MP3.3 ?
| B0015 (double terminator B0010-B0012) - FV
| 4
| EcoRI and XbaI
|
| MP119
| promoter J23101 - BV
| 1
| SpeI and PstI
|
| MP104
| PTet (Tet promoter) - BV
| 1
| SpeI and PstI
|
| MP114
| TetR - FI
| 1
| EcoRI and SpeI
|
| MP143
| gfp generator - BI
| 2
| SpeI and PstI
|
We had a problem with a gel and we lost these digestions.
|
 "
"

