Team:Bay Area RSI/Project
From 2008.igem.org
| You can write a background of your team here. Give us a background of your team, the members, etc. Or tell us more about something of your choosing. | |
|
Tell us more about your project. Give us background. Use this is the abstract of your project. Be descriptive but concise (1-2 paragraphs) | |
| Team Example 2 |
| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Notebook |
|---|
(Or you can choose different headings. But you must have a team page, a project page, and a notebook page.)
Abstract
Every year over 1.2 million people suffer myocardial infarction (MI). The resulting heart damage requires new approaches for effective repair. Stem cell therapies provide hope. However none of the stem cell therapies currently in clinical trials addresses the need for efficient stem cell targeting to cardiac tissue or the need to replace efficiently dead tissue with new cardiomyocytes. To address these problems, we have built several genetic circuits that work sequentially to repair the heart. First, we have built an inducible differentiation circuit that closely resembles the endogenous differentiation pathway, to program cells to become cardiomyocytes. Second, we have built circuits that use the extracellular domains of chimeric proteins to target cells to damaged cardiac tissue. Upon binding, novel receptor-coupled intein-mediated signaling domains activate effector genes that then aid in integration, inhibition of cell death, and the alteration of the tissue microenvironment.
Introduction
There are many problems that existing therapies, and those currently in clinical trials, continue avoid addressing. Some of the main issues include: how to recognize damaged heart tissues, how to deliver stem cells to areas beneath the surface, the inability of adult stem cells to differentiate into cardiomyocytes in the absence of external cues, the prevention of stem cell death during therapy, and how to stimulate advantageous pathways for new cardiac cell integration. Because all our current therapies fail to address any one or a combination of these problems, cell-based therapies for MI patients do not work very well and provide only modest improvement in function, if any.
In 2007 the RSI Bay Area Consortium Team designed and engineered novel methods of targeting damaged cardiomyocytes. Since then, we have shown that our targeting circuit for damaged cardiomyocytes binds and relays effectors in rat cardiomyoblasts. To complement this circuit, the 2008 RSI Bay Area Consortium Team designed and created an efficient inducible method for the differentiation of cardiomyocytes. Together, the two circuits resolve many of the problems associated with the current therapies for MI patients.
Targeting Infarcted Cardiac Tissue
To target cells efficiently to damaged cardiac tissue, the 2007 RSI Bay Area Consortium Team choose three different targets: C-reactive protein (CRP), ICAM, and myosin heavy chain. From these three targeting systems, we focused on continuing the characterization of the CRP based intein system.
CRP As A Target of Infarcted Cardiac Tissue
During an MI, the inflammation causes a several fold increase in CRP serum levels as well as the generation of CRP by damaged cardiomyocytes. However, it is the deposition and immobilization of serum CRP onto exposed small nuclear ribonucleoproteins of infarcted cardiac tissue that makes it an ideal target for therapeutic applications. Because nuclear ribonucleoproteins are contained intracellularly, and only exposed upon serve cellular damage, there is minimal or no non-specific binding of the receptors.
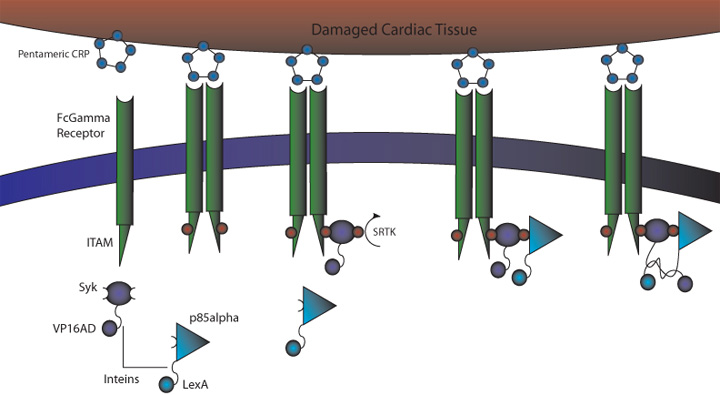
C-Reactive Protein Intein Mediated Receptor Signaling Circuit
1) MI induces large increase in serum CRP levels. It also induces CRP expression in cardiomyocytes. 2) CRP deposits onto damaged myocardial cells (it binds to exposed small nuclear ribonucleoproteins on dying cells) 3) Our cells expressing FcGammaRIIa-R131 bind CRP on the surface of damaged cells and in the serum surrounding the damaged area. 4) Crosslinking of the receptors induces the phosphorylation of the ITAMs in the intracellular region of the receptor. 5) Syk is recruited to the receptor and becomes phosphorylated at ITAMs. 6) Crosslinking of the receptors also recruits p85 which binds to the phosphorylated Tyr-317 residue on Syk. 7) This interchange allows for the inteins (dnaEc-VP16AD at C terminus of Syk and dnaEn-mLexA at N terminus of p85) to interact and self-splice. 8) Self splicing of the inteins produces the VP16AD-mLexA needed for downstream signaling.
Benefits: Binds to CRP on damaged cells to allow for targeting of therapeutic agents (versatile since CRP is expressed in a variety of inflammed tissues). Our system also removes CRP from the region surrounding the damaged area, lessening the damage caused by CRP and increasing the chance of full recovery.
Problems: FcGamma can bind IgG present in serum. Although the FcGammaRIIa-R131 exhibits weak affinity for IgG the possibility of binding and crosslinking the receptor is still there. This problem can be alleviated if the the FcGamma-CRP system is used as part of an AND gate with another receptor system.
FCGamma Receptor Binds Immobilised CRP on Damaged Cardiomyocytes In Vitro
description picture picture exp
CRP activates GFP signaling upon FCGamma Binding
picture picture exp
Generation of Clonal lines of FCGamma+ rat H9C2 Cardiomyocytes
pic + explanation
Effectors To Aid in integration, anti-apoptosis, and the alteration of the tissue microenvironment
Overview Construct image pics from slides
CRP Targeting Circuit Concerns
Future Directions
Sensor sensitivity-> , avidity, affinity More universal receptor --> 2nd gen SCFV to target other tissue
Cardiomyocyte Differentiation Circuit
endogenous pathways pathway overview construct image + explanation data
Differentiation Circuit Concerns
CA, efficiency, transdiff,
Future Directions
inducible, non-SC progenitor, native cardiofiobroblast
Use As a Novel Therapy For MI Patients
Current therapies and problems Diff types of MI and need for better solution Therpeautic approach with combined circuitry use in tandem, synergistic application
 "
"