Team:Newcastle University/Original Aims
From 2008.igem.org
Newcastle University
GOLD MEDAL WINNER 2008
| Home | Team | Original Aims | Software | Modelling | Proof of Concept Brick | Wet Lab | Conclusions |
|---|
Home >> Original Aims
Original Aims
The standard practice in biology today, synthetic or not, is to treat bioinformatics as any other tool to achieve an end. A problem is determined, and the biologist works out a possible avenue of exploration. Sometimes this involves the use of bioinformatics tools, such as BLAST searches or phenotypic trees. Once the method of exploration is established, the biologist rarely goes back to bioinformatics approaches to analyze her results.
We consider bioinformatics to be not only a tool in biology, but as a viable but limited method for exploring a problem. Wet-lab biology is expensive in terms of time, money, and manpower. A single bioinformatician can test the same situation with no equipment other than a computer, and run many iterations of the same experiment within seconds, rather than the weeks that the labs may take.
However, the bioinformatics is only as good as its simulation. The field expands daily with new information, all of which must be incorporated into the simulation in order for it to give useful results. Much of bioinformatics is backed by hard data from wetlabs. Running an experiment 1000 times is only useful if you know what all the variables are, and what they should be.
The most sensible approach to the problem of these different yet complementary methods is to play one off of the other, taking advantage of the strengths while minimizing the limitations.
BugBuster
We aimed to develop a diagnostic biosensor for detecting pathogens. We wanted this to be cheaply and readily available for deployment in areas with limited medical resources such as refrigeration and sophisticated laboratories. We chose to use Bacillus subtilis as a method of delivery due to its ability to sporulate. The sensor bacteria could then be dried down as spores, which are extremely resilient to environmental conditions, and then could be rehydrated when required.
Our aim was to develop a strain of Bacillis subtilis 168 with the ability to detect four Gram-positive pathogens through their extracellularly secreted quorum-sensing peptides, and indicate through reporter genes linked to the receptor-ligand cascade which of these are in its growth medium.
Gram-positive bacteria communicate using quorum communication peptides. Research has shown that they are extremely strain-specific. Our B. subtilis has a range of genes engineered that enable detection of these peptides. The issue that we had to overcome is the fact that we have more quorum-sensing peptides to detect than fluorescent proteins to show their presence. The output of the system is followed by analysing expression of fluorescent proteins such as mCherry, GFP, CFP and YFP.
Mapping multiple inputs to three output states is a multiplexing problem. The design of the genetic circuitry to do this is non-trivial and is not feasible manually. We therefore chose to use a biological implementation of an artificial neural network (ANN). Our team members wrote designed and implemented a complete suite of tools that allowed the design and simulation of regulatory networks. An essential part of the approach was the use of computational evolution to design circuits with predictable behaviour even when the details of the required topology are unknown in advance. We used the modelling language CellML because of its ability to easily model virtual parts that can be easily assembled into a circuit which can be simulated. We aimed to translate this model into a sequence that could be implemented as BioBricks.
A visual output was chosen for detection of pathogens as this allows for rapid detection without a requirement for specialist equipment. The aim was to have different fluorescent protein outputs relating to the presence of different pathogenic bacteria, and different combinations of these bacteria. In line with the neural network concept of the evolutionary algorithm, we wanted to map numerous inputs to limited outputs. Four pathogens can be detected; we wanted different outputs for each of these and each combination of these.
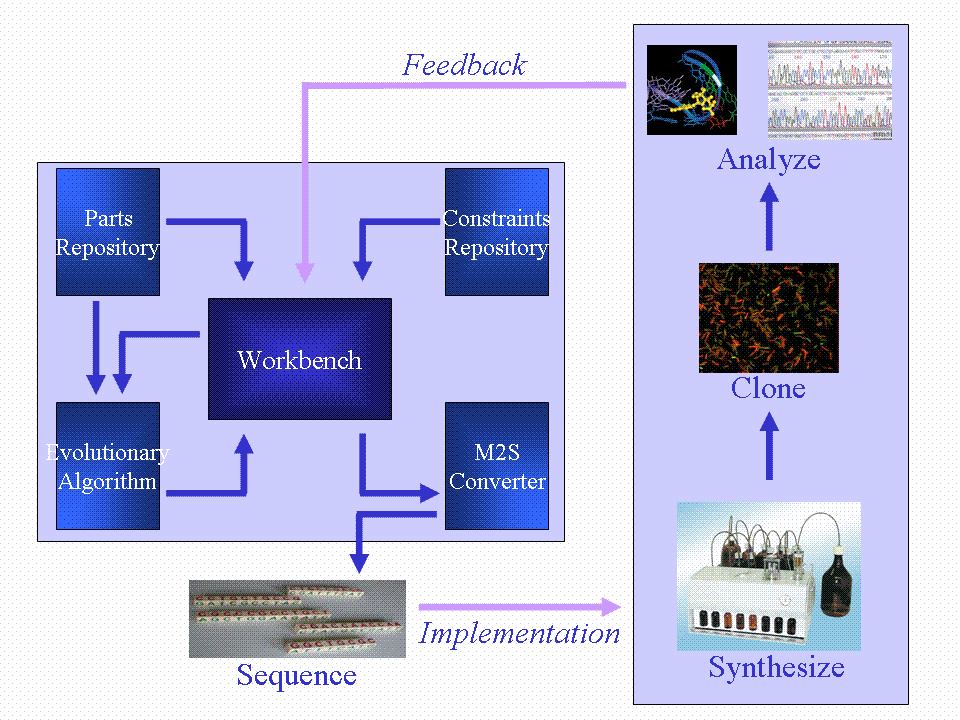
We will produce a workbench that will incorporate a parts repository, constraints repository and an evolutionary algorithm (EA). The EA will take input from the parts repository and constraints repository to evolve a neural network simulation. The fittest model will be used to generate a DNA sequence which will implement the neural network in vivo. This DNA sequence will be synthesized and cloned into the B. subtilis chassis. One of our outcomes will be a range of neural network node BioBrick devices which can be combined to form the in vivo neural network.
In keeping with the neural network model, our bacterial circuit would have three layers of complexity. The plan can be summed up as follows:
- The input layer to the bacterial neural network would be represented by the two-component genes, activated by the peptides from the four gram-positive pathogens. Each peptide would represent a node. We shall refer to them as parts.
- The hidden layer would represent an assortment of different transcription factors from the different pathogenic bacteria.
- The output layer would represent three fluorescent proteins; GFP, YFP and mCherry.
The biologist would specify the inputs and the outputs; i.e. in the presence of said peptide, said fluorescent protein will light up.
This was the starting point from which the construct was designed.
 "
"