Team:Chiba/Demo experiments
From 2008.igem.org
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
Contents |
Demo Experiment ~Senders~
Method
- Pre-culture
- Picked and cultured the following glycerol stocks in 2mL of LB:
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (BW)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), (XL10G)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]), (XL10G)
- Cultured at 37°C for 12h.
- Picked and cultured the following glycerol stocks in 2mL of LB:
- Culture
- Added 6.25% each of the pre-cultures to new LB medium.
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)])
- Cultured at 37°C for 4~5h。
- Added 6.25% each of the pre-cultures to new LB medium.
- Wash
- Transfer 10mL each of the culture to 50mL centrifuge tubes.
- Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant.
- Added LB-Amp to each centrifuge tube:
- 10mL to the tube that contains [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- 5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 10mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Mix
- Mixed the sender cells [http://partsregistry.org/Part:BBa_K084012 BBa_K084012] and [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] both with [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] at a 1:1 ratio.
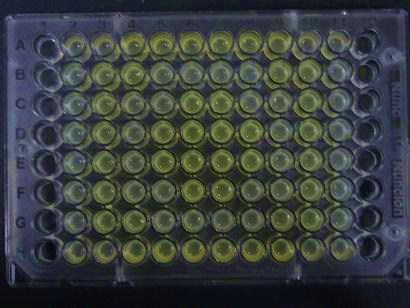
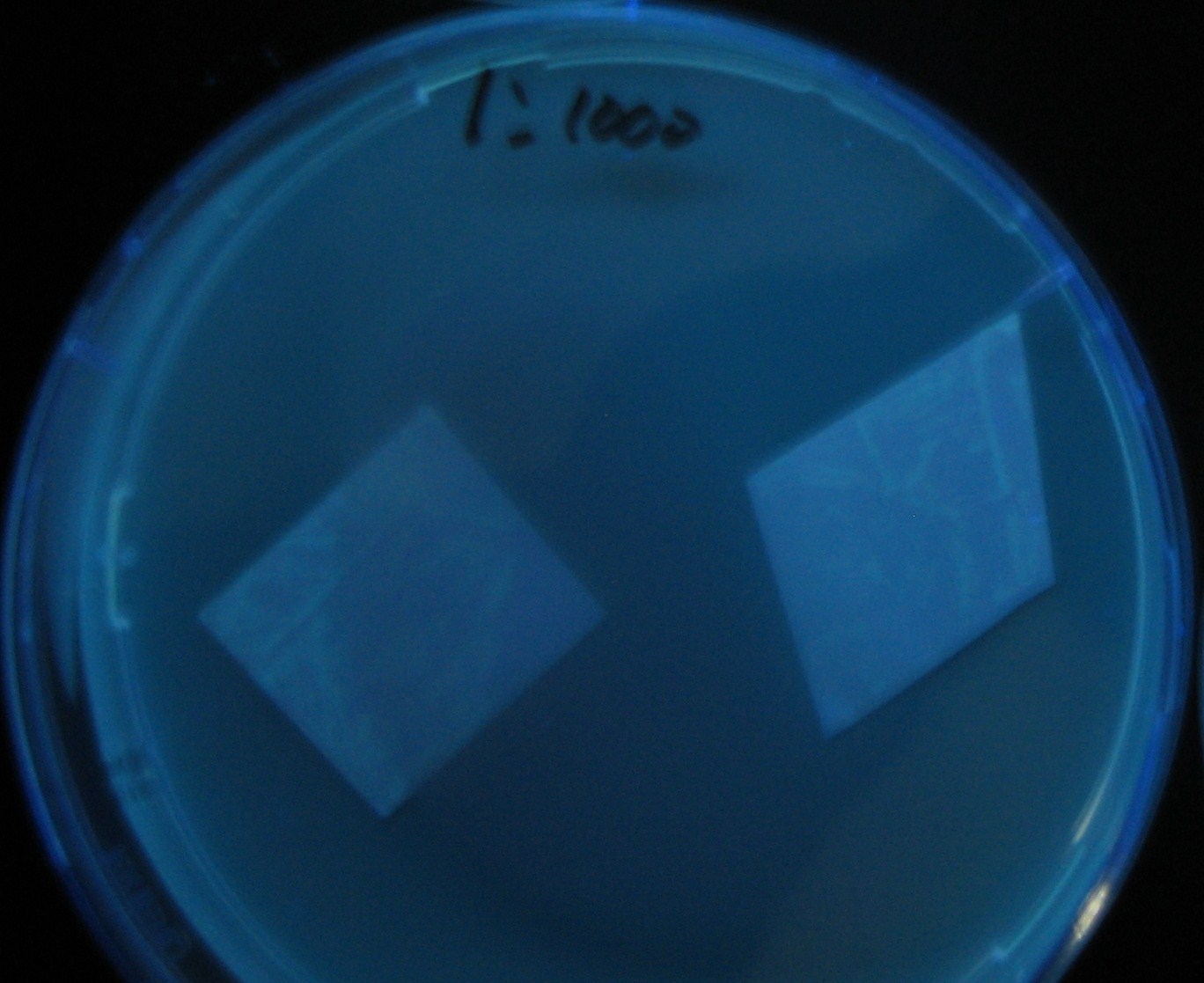
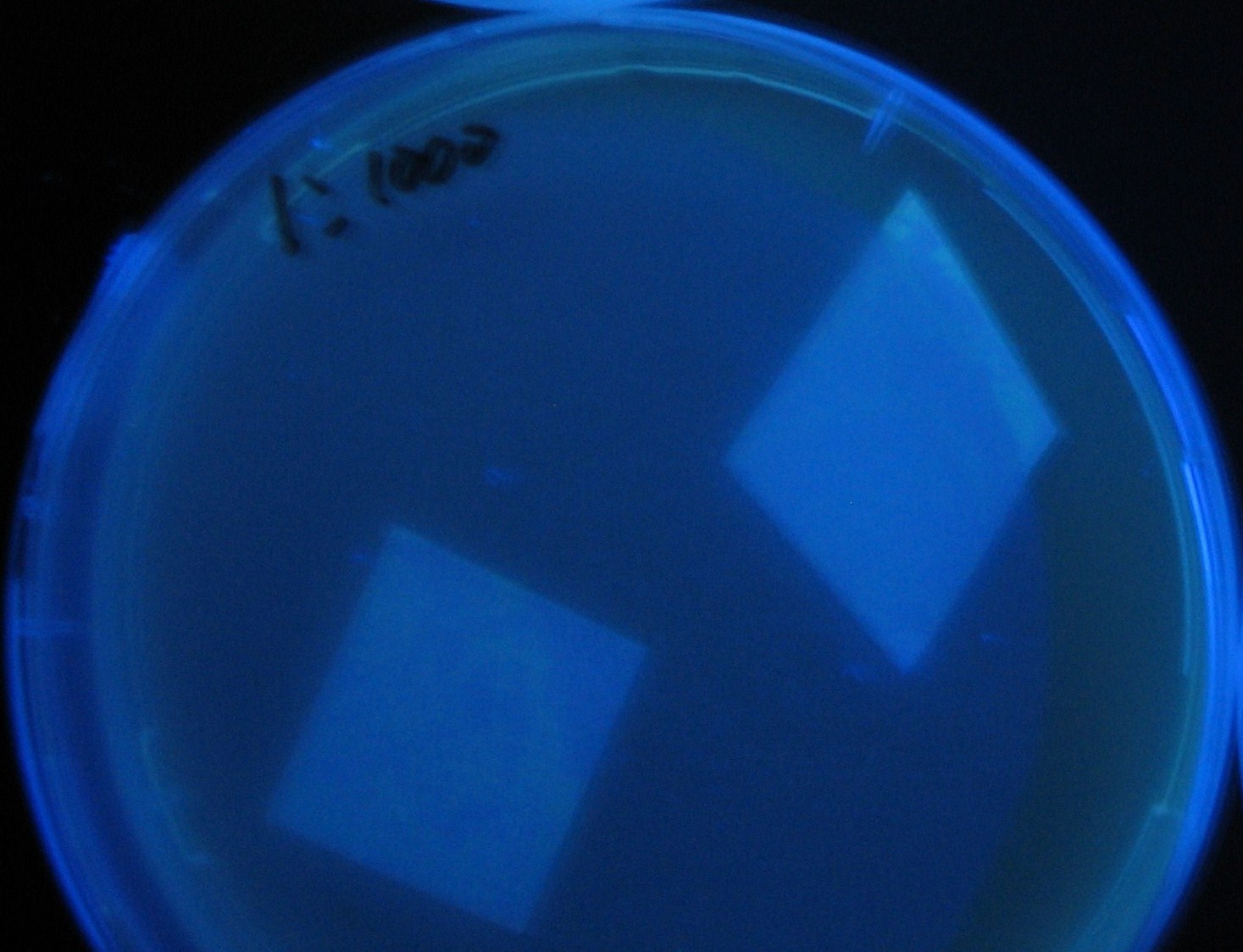
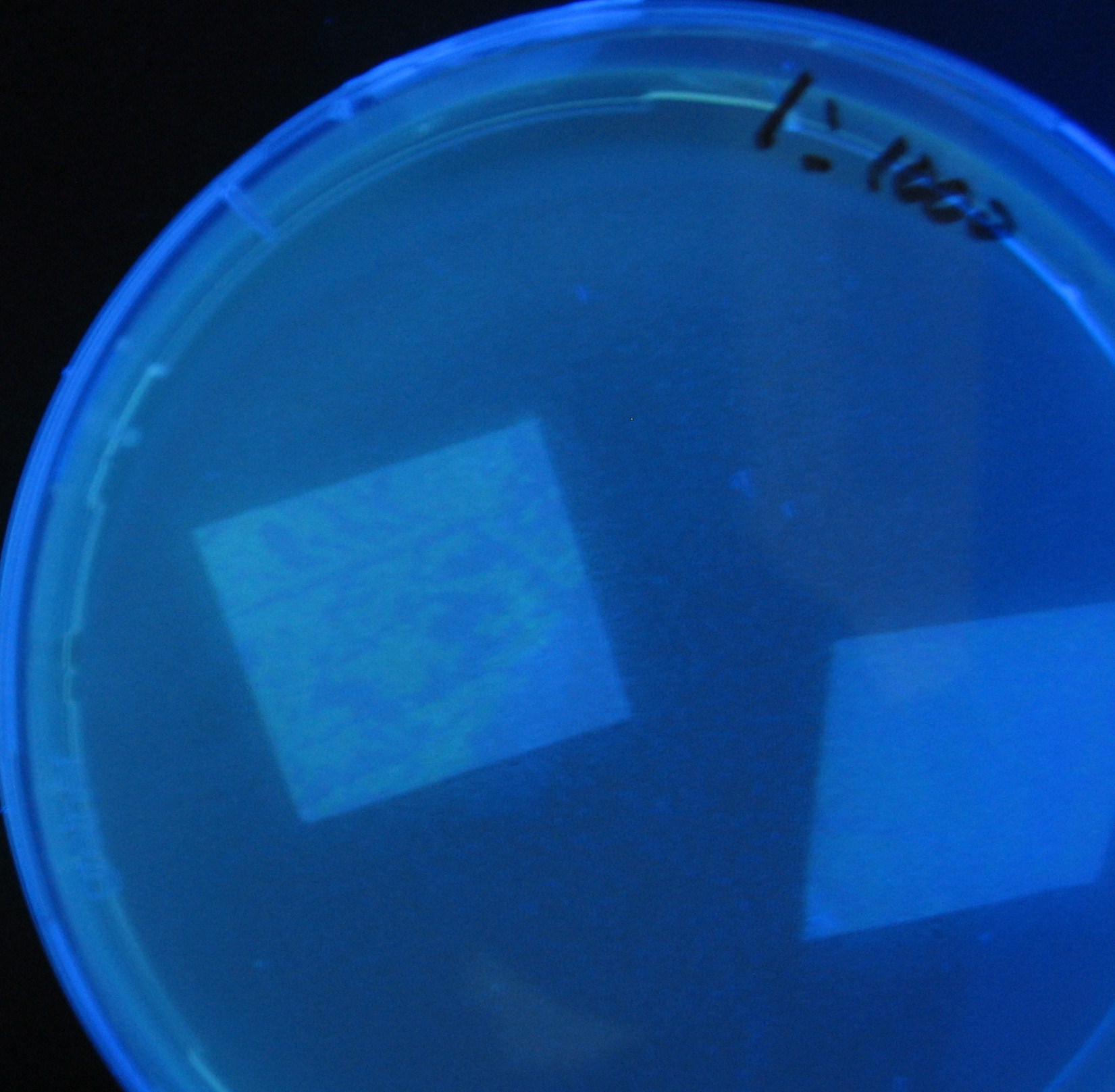
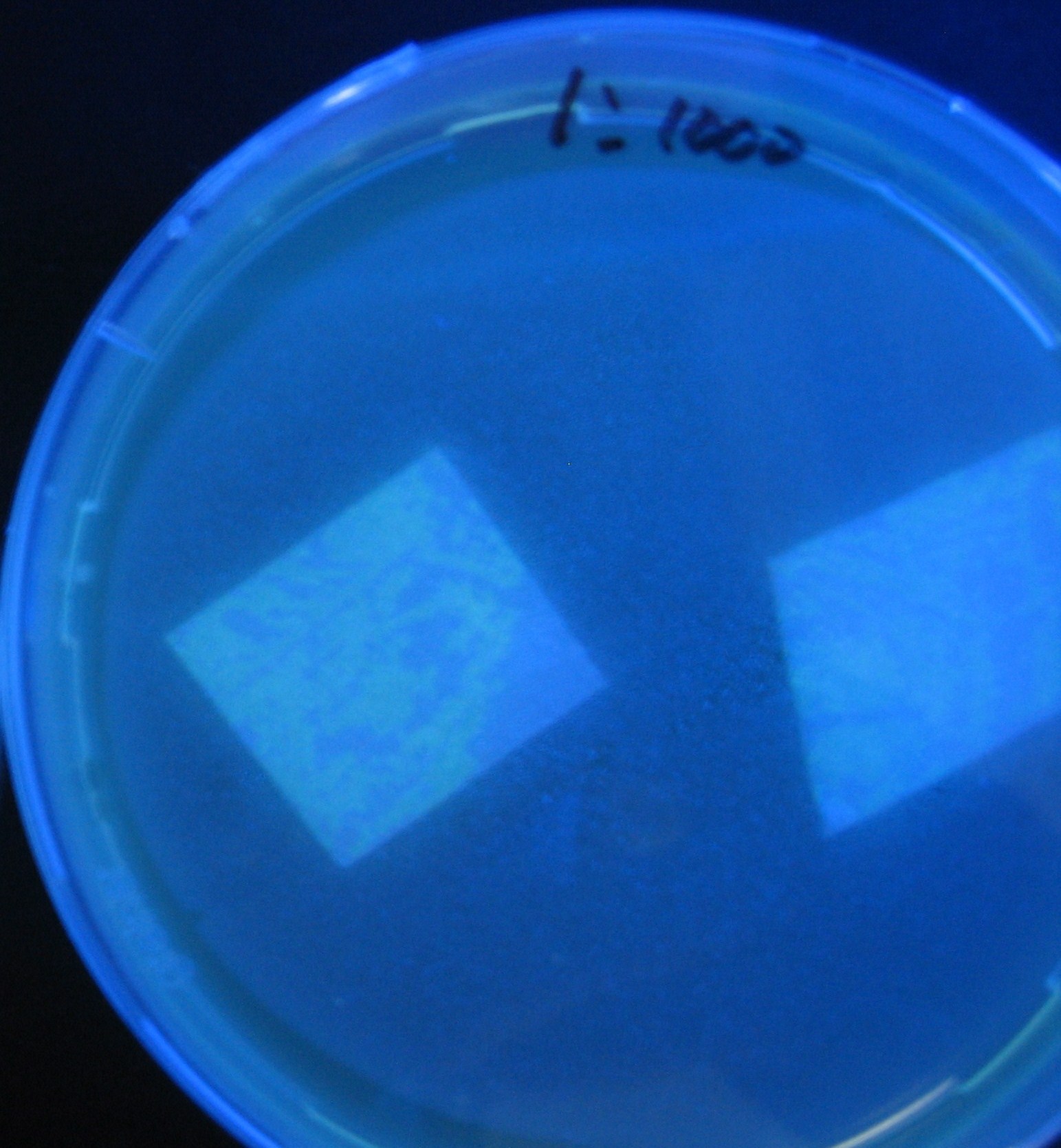
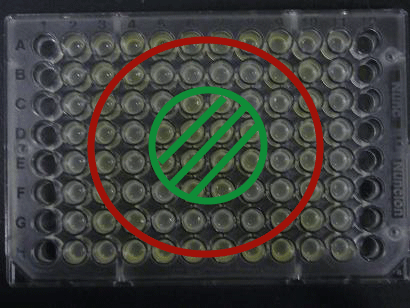
- Added 100μL each to a 96-well shallow plate (as shown in the figure).
- Green part is[http://partsregistry.org/Part:BBa_K084012 BBa_K084012]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Red part is [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Uncolored part is [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] alone.
- Culture and observe results
Results
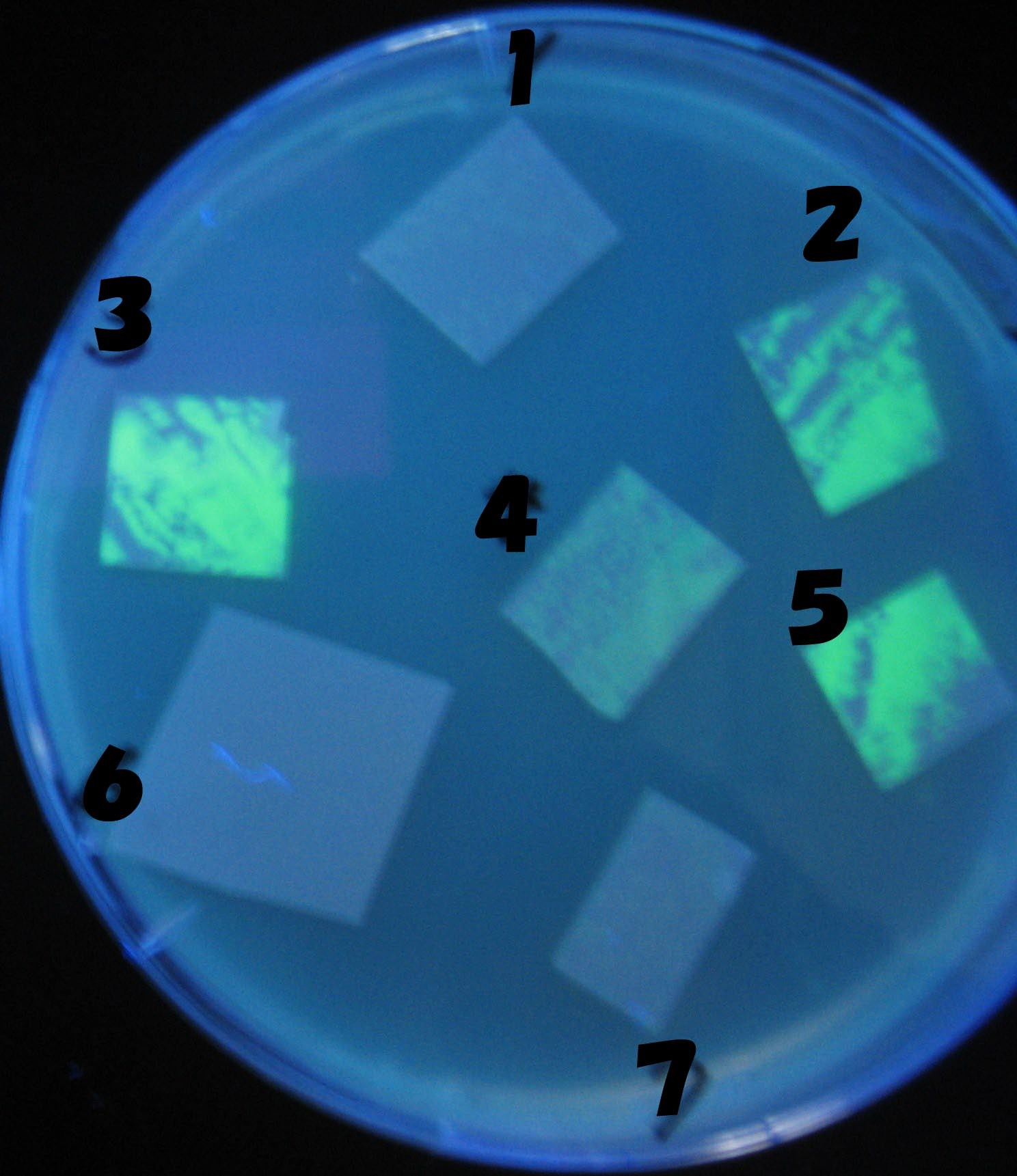
 Green region: sender=LuxI,
Red circular region: sender=Las I.
Green region: sender=LuxI,
Red circular region: sender=Las I.
LuxI GFP is detected at 4h following mixing while LasI GFP is detected
after 8h, thus successfully demonstrating time-delay depending on the
sender used.
--Yoshimi 13:41, 29 October 2008 (UTC)
Demo Experiment ~Receivers~
Varying bacterial numbers: method
- Receiver(T9002) pre-incubation
- Receiver:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW)wascultured in 2mL LB-Amp (37°C,12h)
- Pre-incubated Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW))was plated so as to produce about 1000 colonies.
- Sender(S03623) pre-incubation
- Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) was cultured in 50mL entrifuge tubes in 10mL of LB-Amp (37°C,12h)(2tubes)
- Sender Wash
- Centrifuged 2 tubes containing([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW))at 20°C,3600rpm for 6min and discarded supernatant.
- Added 10mL LB-Amp to each tube.
- Repeated wash twice.
- Creating bacterial plates
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) tube 1 (10mL) was mixed with LB-Amp-agar(50°C)(10ml)to produce sender containing bacterialplate-1.
- LB-Amp pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) tube 2(100μl)was mixed with LB-Amp(9.9ml) and diluted 100-fold. 10ml of this solution was mixed with LB-Amp-agar(50°C)(10ml) and created Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW))containing bacterial plate-2.
- LB-Amp pre-cultured Sender solution-2(10μl) and LB-Amp(9.99ml)was mixed to dilute1000-fold.10ml of this solution and LB-Amp-agar(50°C)(10ml) was mixed to create Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) containing bacterial plate-3
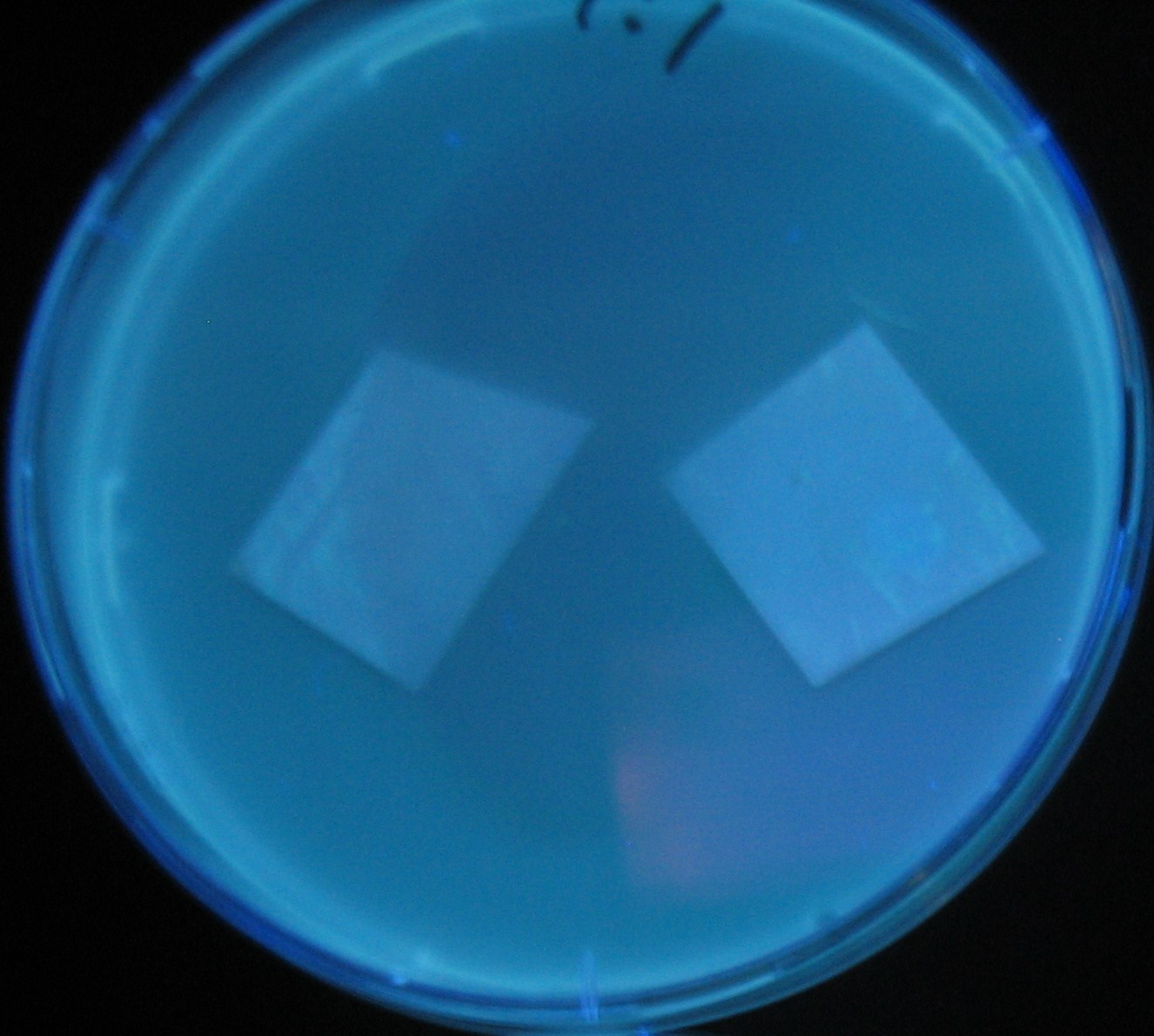
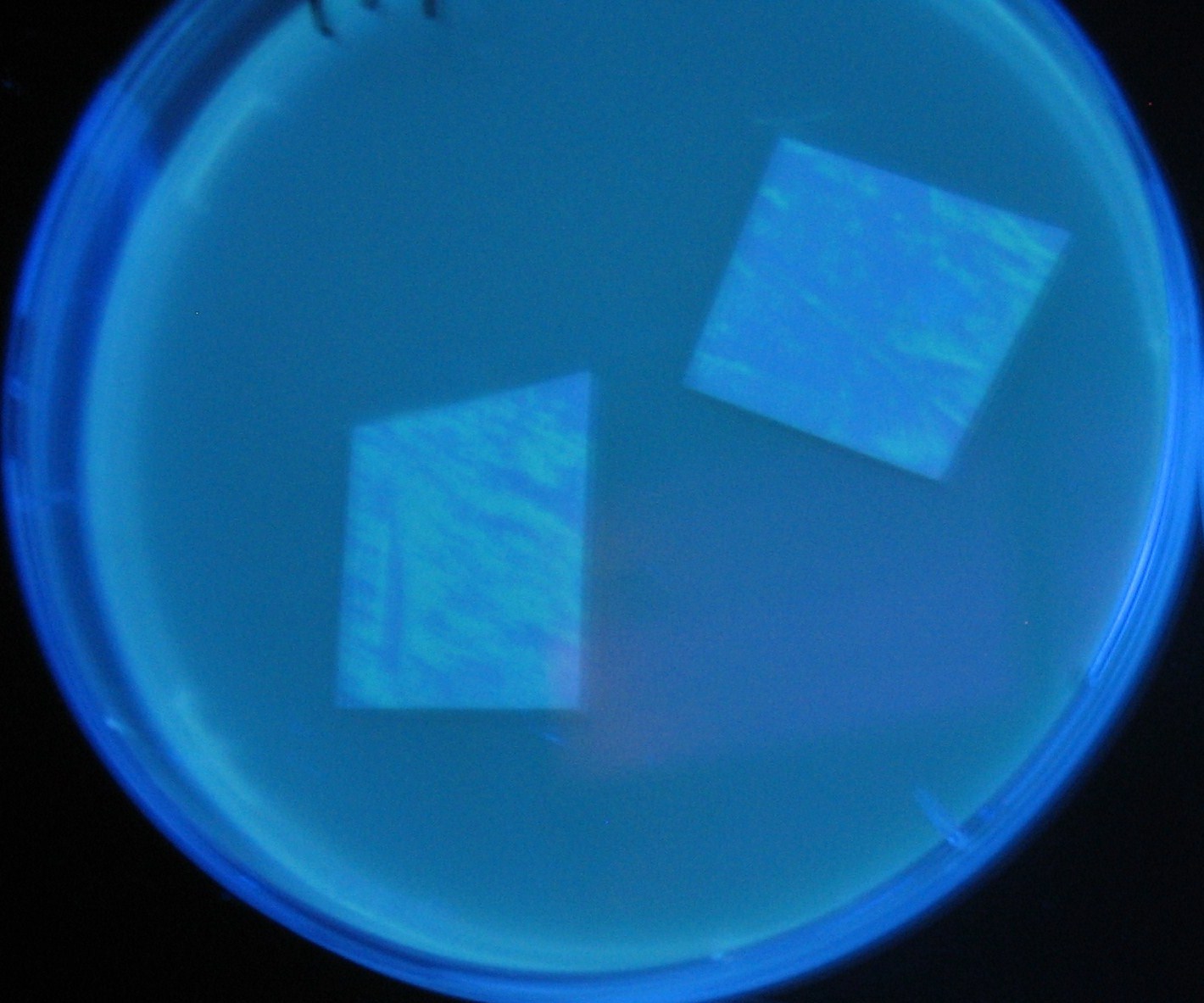
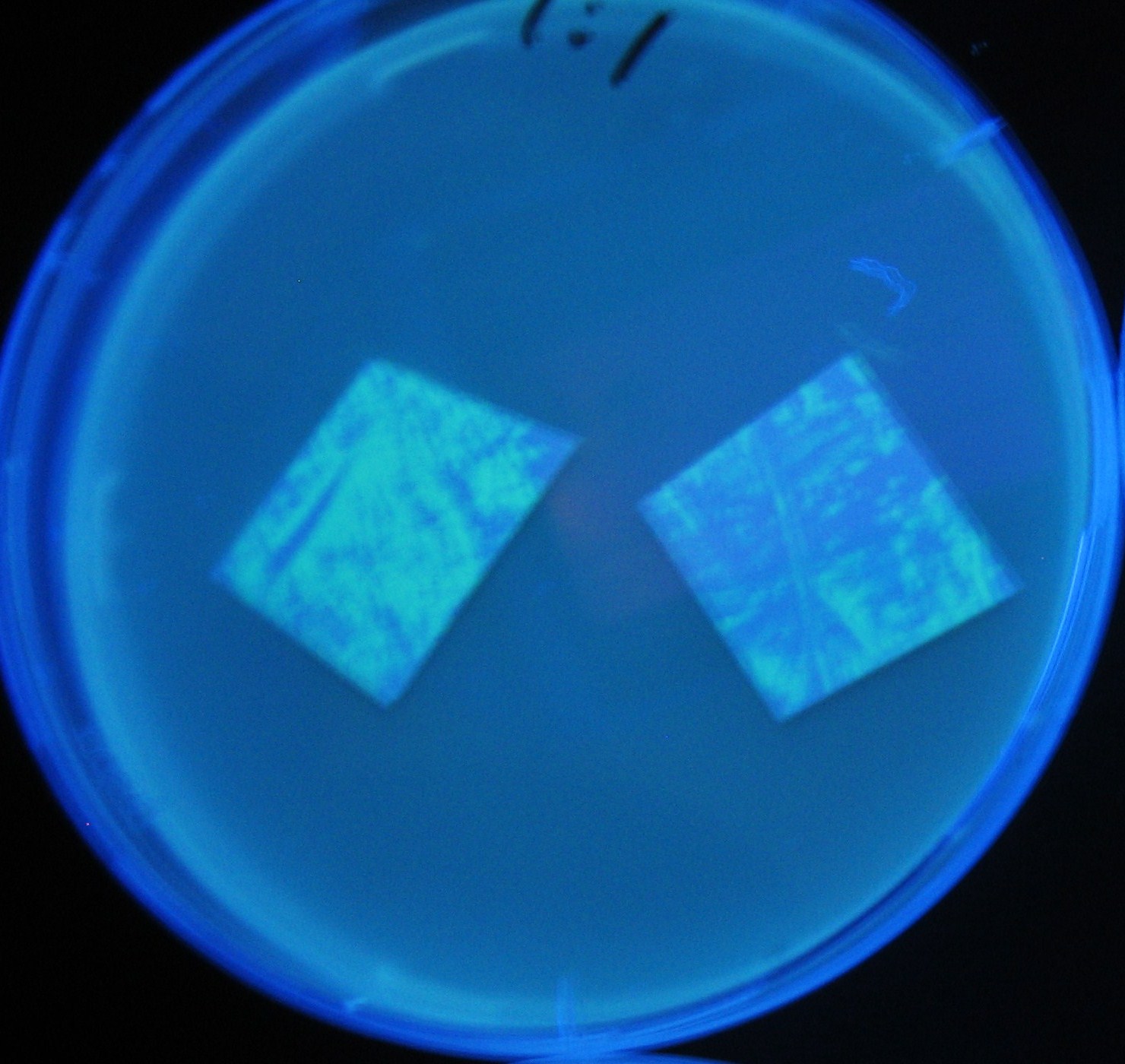
- Lifted with nitrocellulose
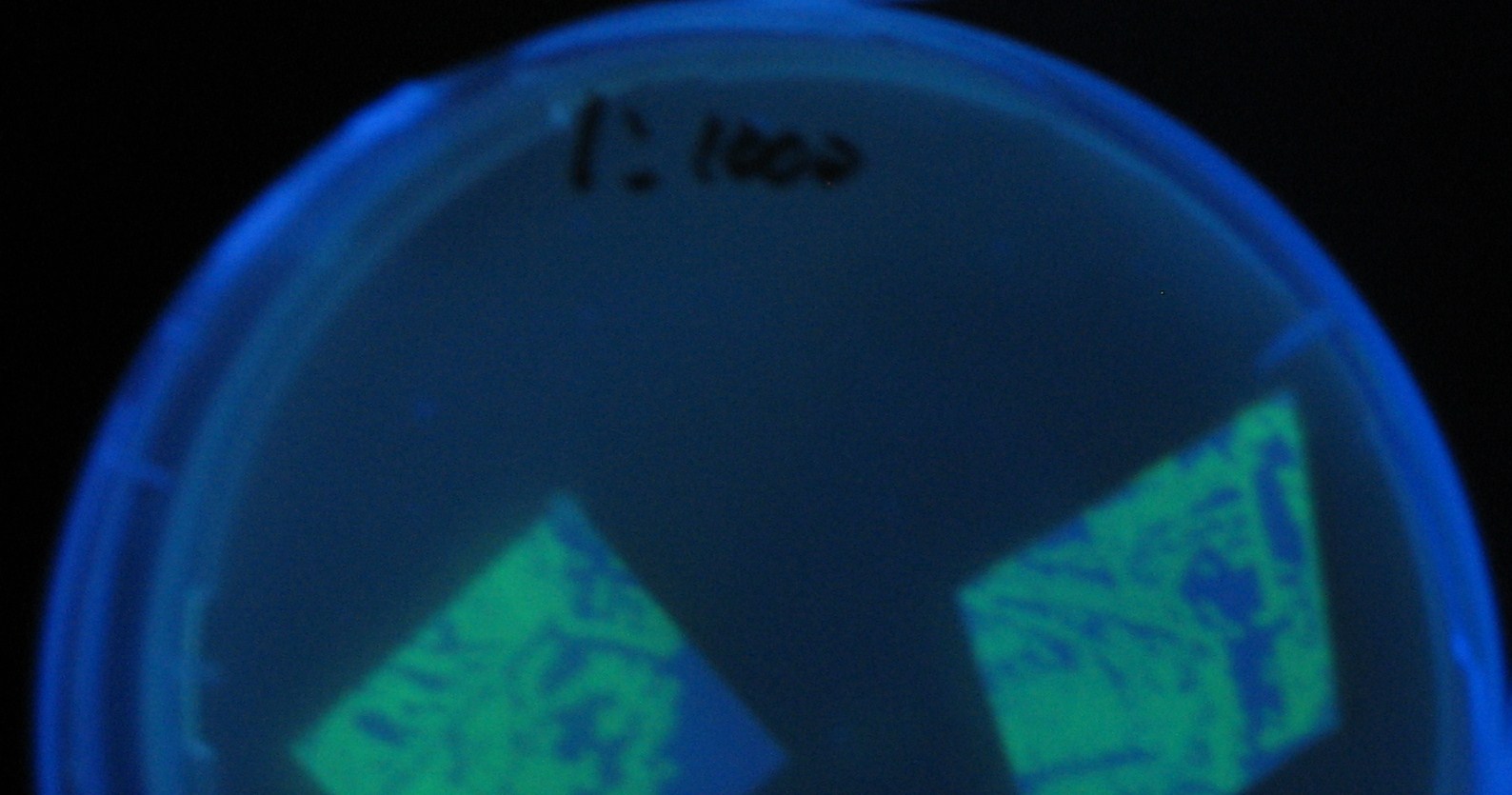
- Receiver([http://partsregistry.org/Part:BBa_T9002 BBa_T9002](BW))colony was transfered to a nitrocellulose filter and placed on each of Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW))containing bacterial plate (1~3) and Sender-absent negative control plate (t=0). Determined the time required for the colonies to fluoresce depending on the bacterial concentration (100 and 1000-fold dilution).
- Method to detect fluorescence
- Plates cultured at 37°C were exposed to UV (312nm) light once every 30 minutes to observe GFP fluorescence.
Testing different receivers-methods
- Receiver&sender pre-culture
- Used Receivers were:
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy)
- ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032]:plux-GFP(high copy)
- Used Receivers were:
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy)
- ptet-mLuxR(too sensitive)-plux-GFP
- ptet-luxR-plux-GFP-plac-aiiA
- (all BW)Each was cultured in 2ml LB (37°C,12h) and plated so that about 1000 colonies of receiver cells will grow.
- Sender:[http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW) was cultured in 10mL LB in 50mL centrifuge tubes (37°C,12h)
- sender wash
- Each receiver-containing medium was centrifuged in 50mL tubes at de20°C, 3600rpm for 6min and supernatant discarded.
- Added 10mL LB to each tube.
- Repeated wash twice.
- Creating bacterial plates
- LB pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) tube 1 (10mL) was mixed with LB-agar(50°C)(10ml)to produce sender containing bacterial plate-1.
- LB pre-cultured Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) tube 2(100μl)was mixed with LB(9.9ml) and diluted 100-fold. 10ml of this solution was mixed with LB-agar(50°C)(10ml) and created Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate-2.
- LB pre-cultured Sender solution-2(10μl) and LB(9.99ml) was mixed to dilute 1000-fold.10ml of this solution and LB-agar(50°C)(10ml) was mixed to create Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate-3
- Lifted with nitrocellulose
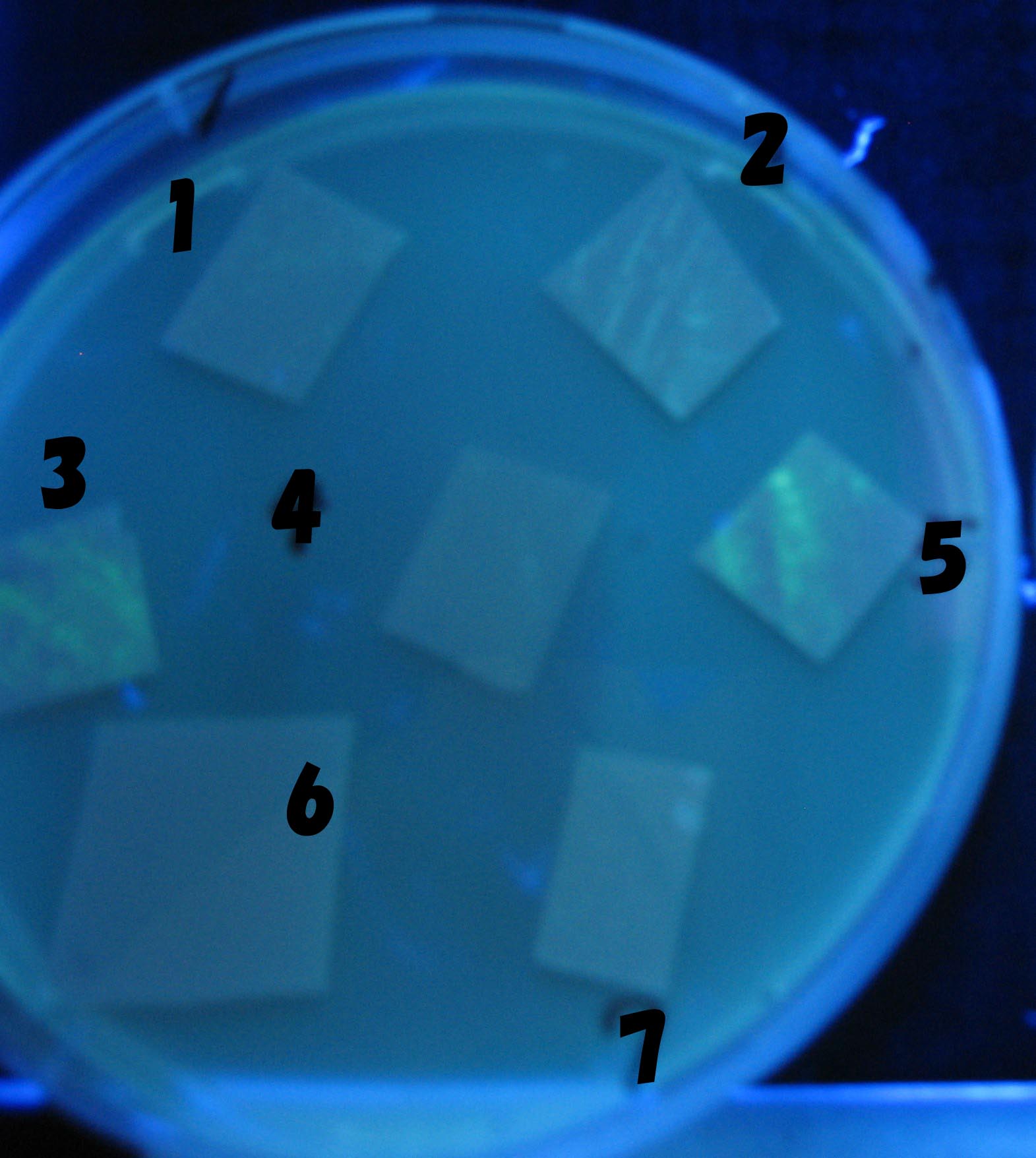
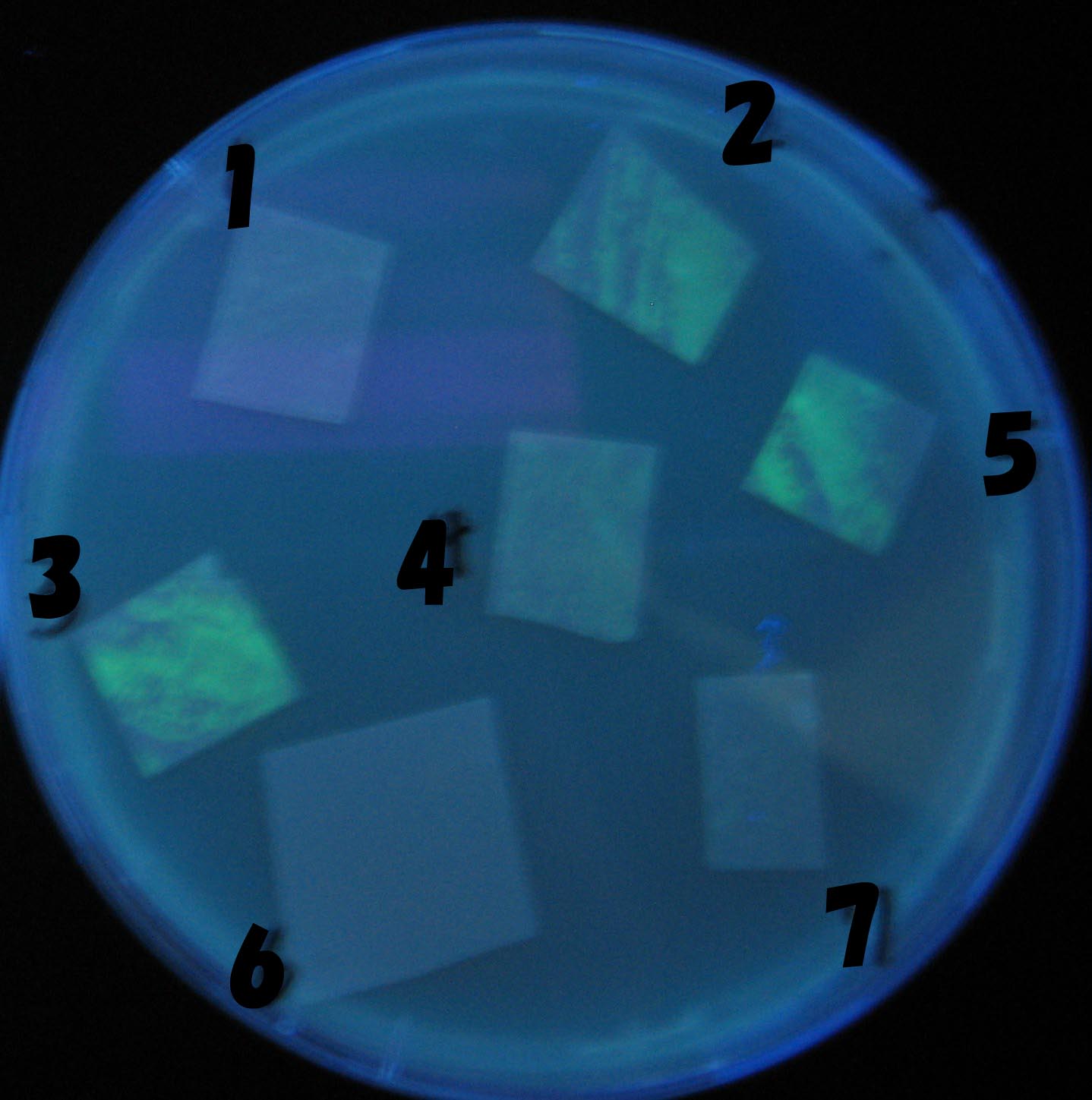
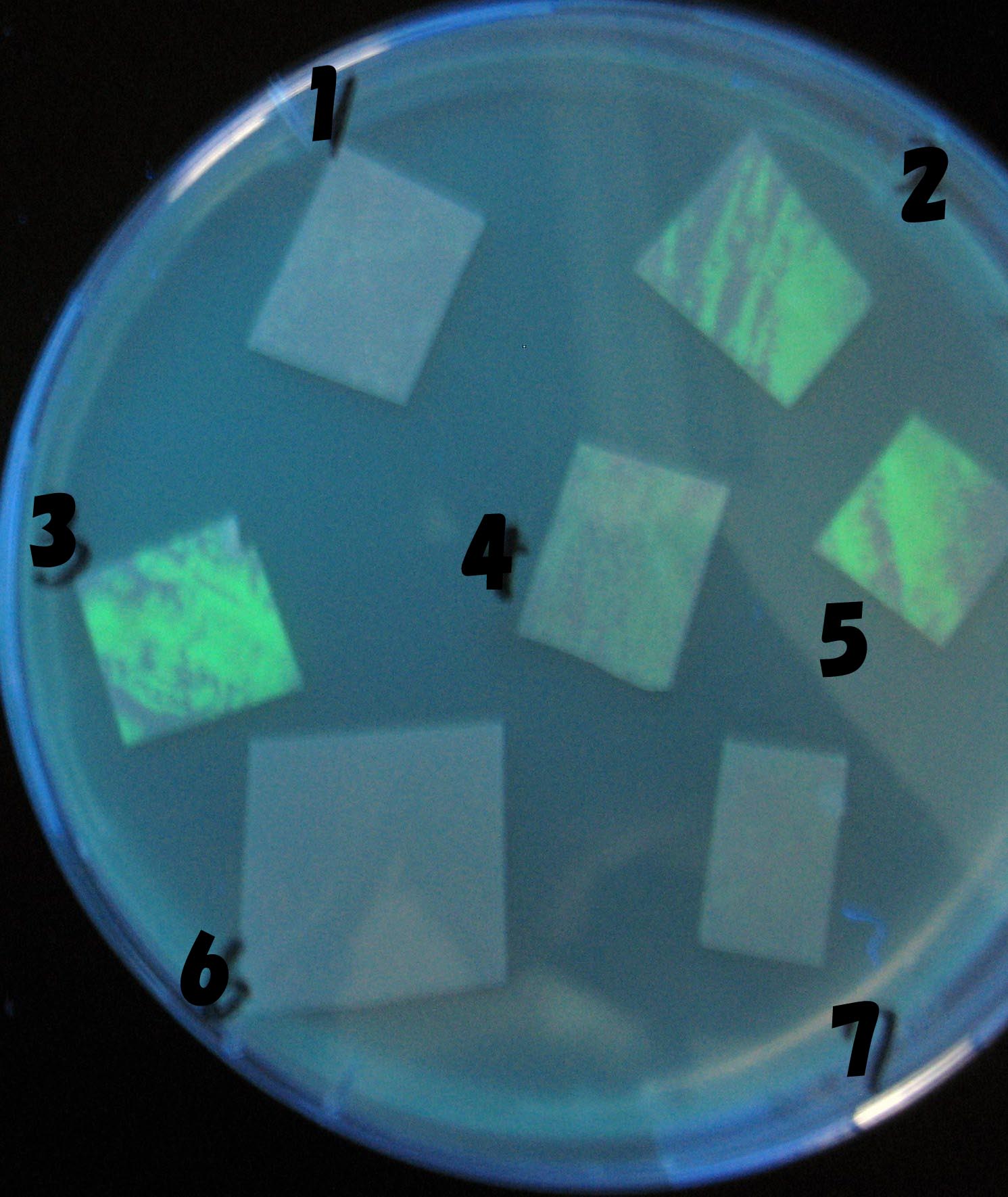
- Each Receiver colony was transfered to a nitrocellulose filter and placed on a Sender([http://partsregistry.org/Part:BBa_S03623 BBa_S03623](BW)) containing bacterial plate (1~3) or a sender-absent negative control plate(t=0) to observe how receiver type affects the time taken for the colonies to display visible fluorescence.
- Method to detect fluorescence
- Plates cultured at 37°C were exposed to UV (312nm) light once every 30 minutes to observe GFP fluorescence.
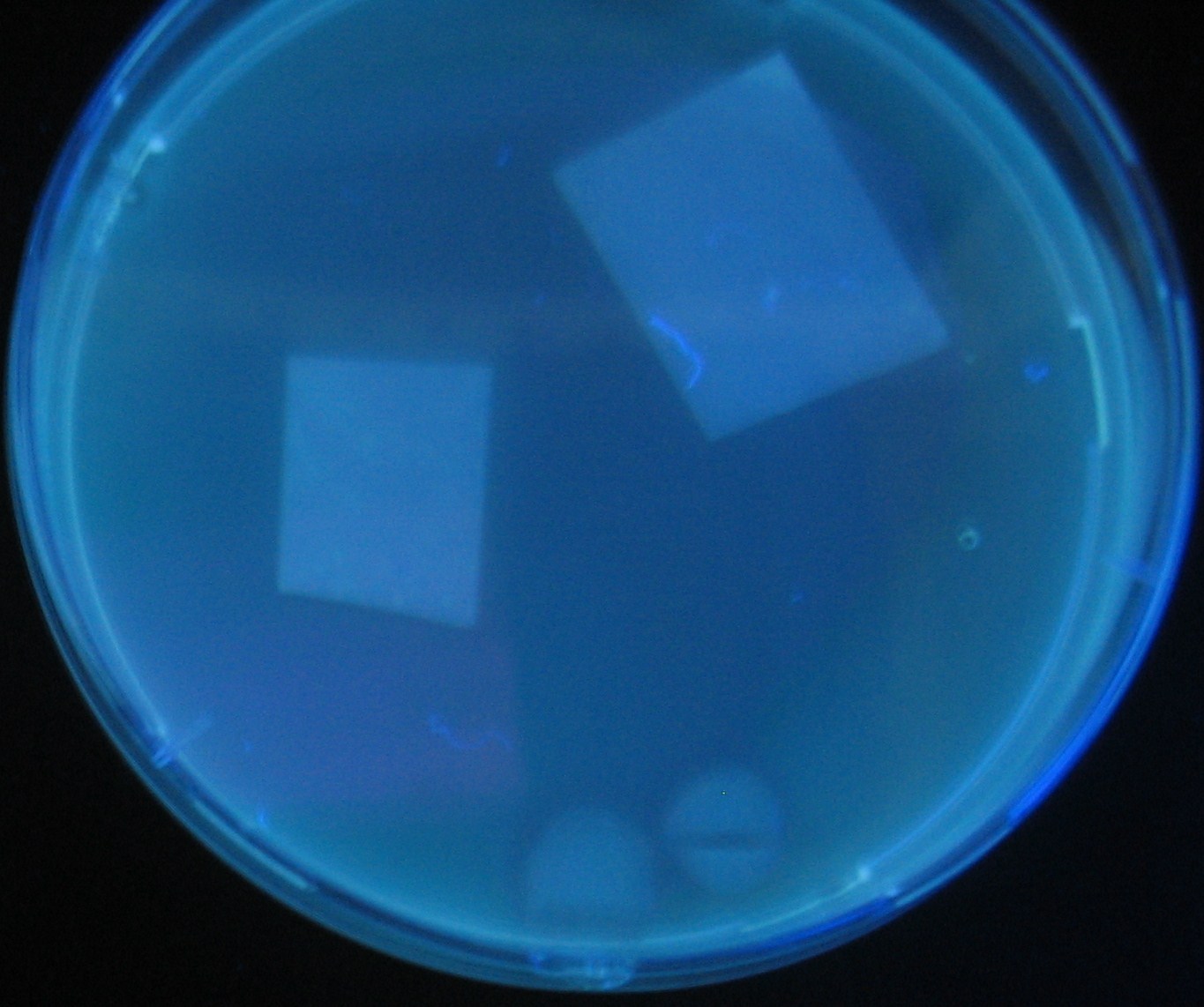
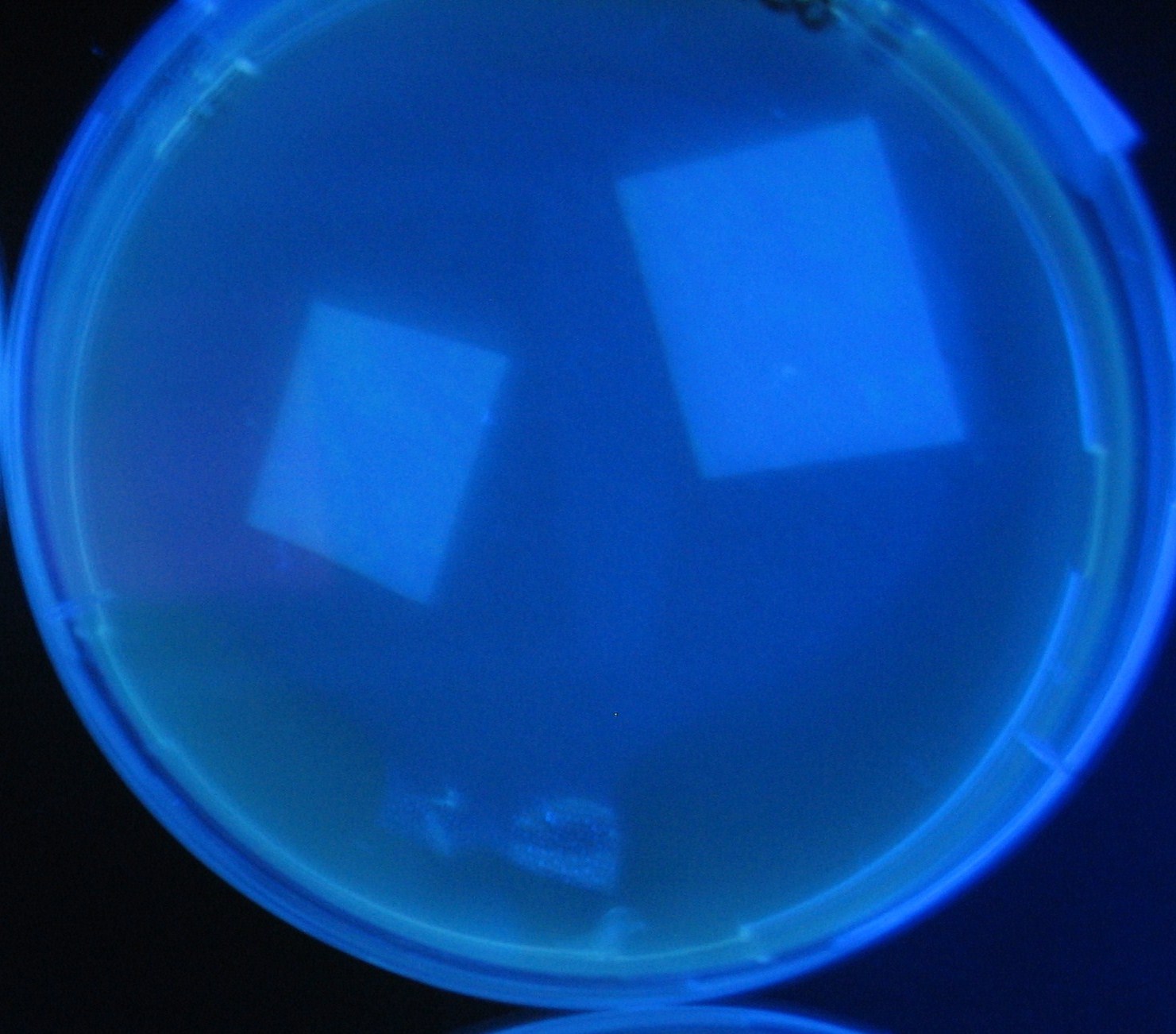
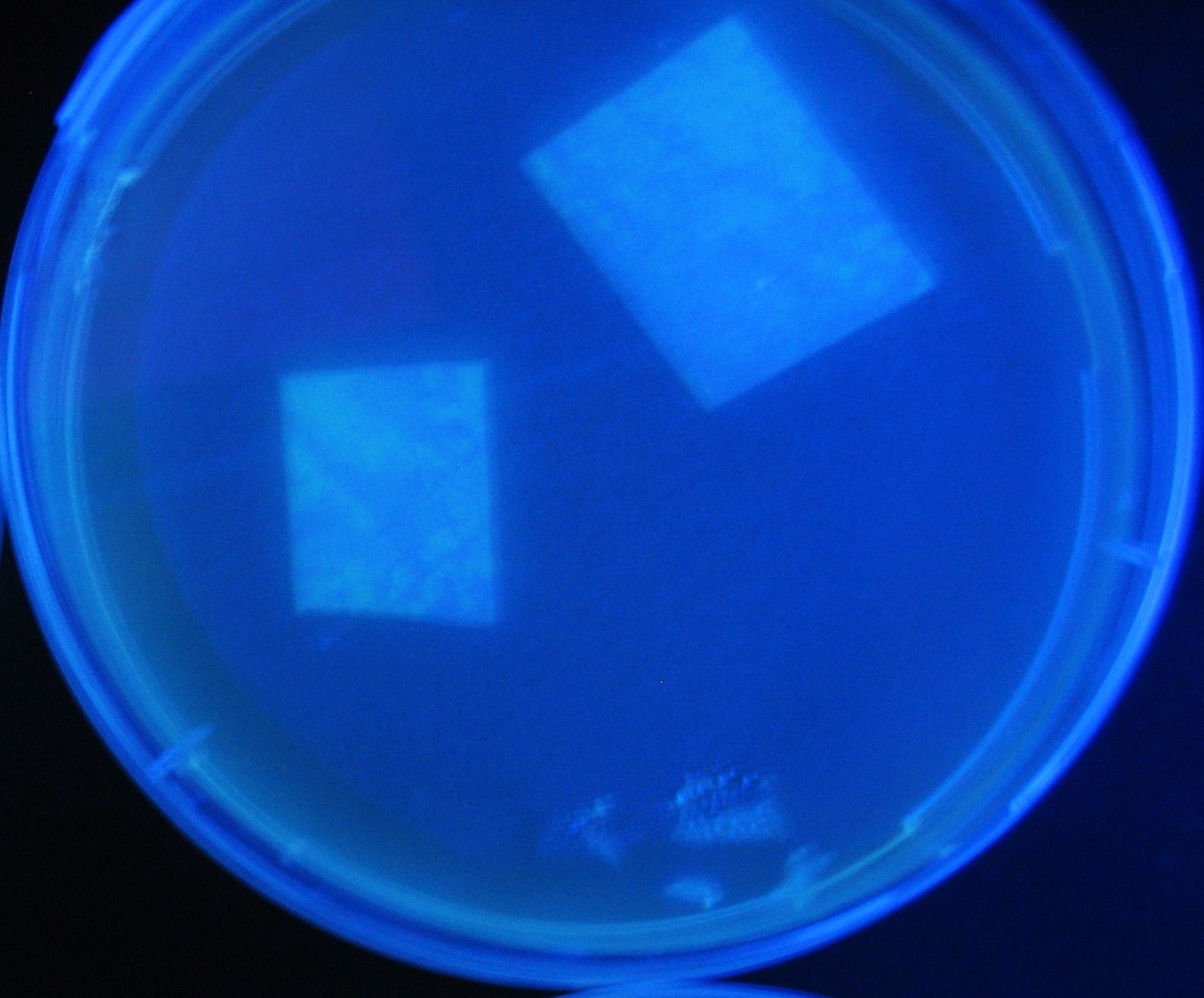
Varying bacterial numbers-results and discussion
results
0h 0.5h 1.0h
0h 0.5h 1.0h 1.5h
0h 0.5h 1.0h 1.5h 2.0h
discussion
菌数を振ることでGFP発現を遅らせることができた。このことから菌一匹が単位時間に生産するAHL量は 菌密度(細胞外のAHL量)に関わらず一定であることがわかる。これはsenderが(AHLによる?)フィードバック 機構を持ってないためと考えられる。 これを利用すれば各レシーバーのGFP発現までの時間を遅らせることができる。(異なるレシーバー間の発現の時間差を広げたり縮めたりすることはできない。)
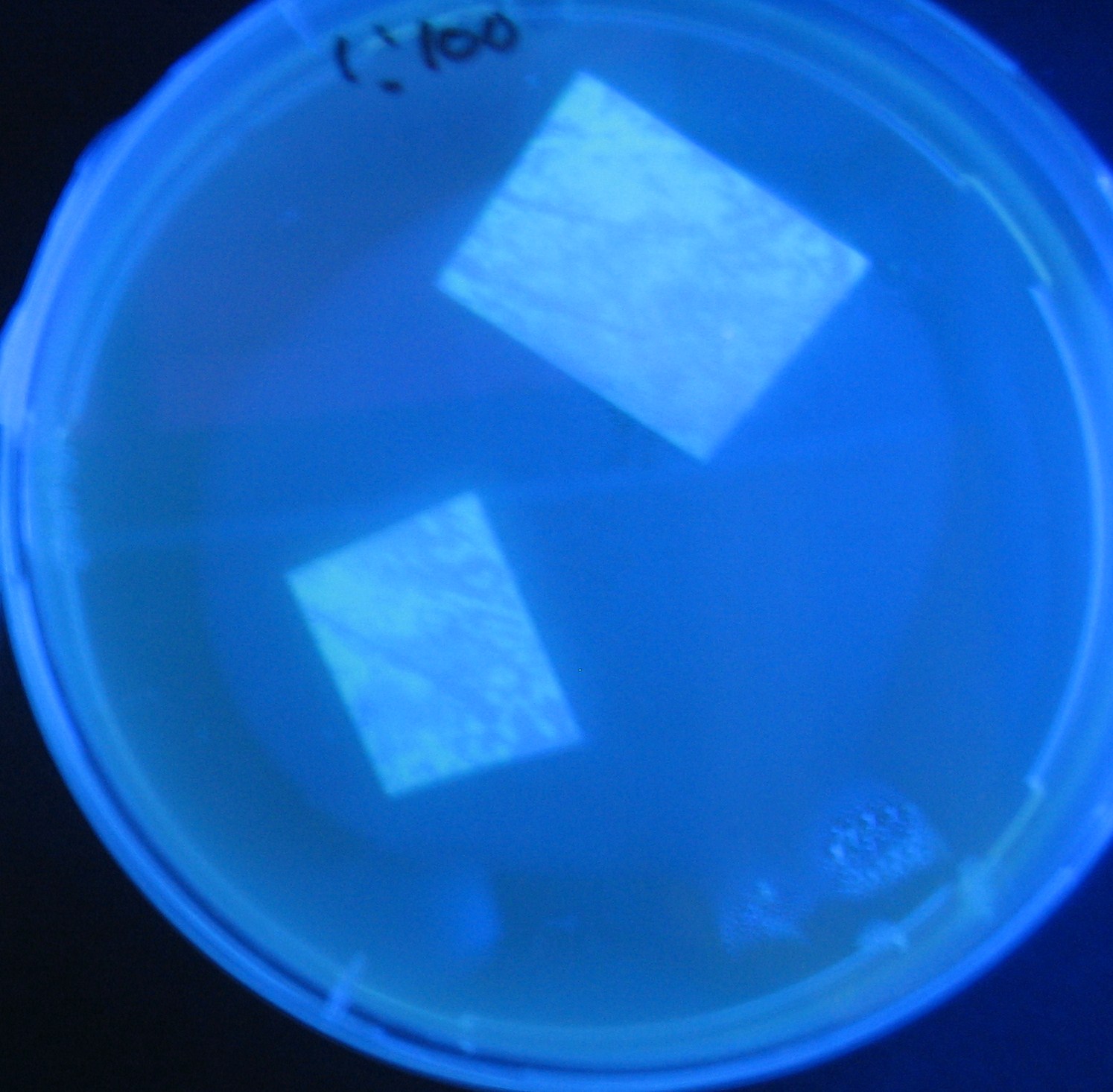
Testing different receivers-results and discussion
results
0h 0.5h 1.0h 1.5h
1=N.C
2=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(high copy)
3=ptet-luxR-(low copy),[http://partsregistry.org/Part:BBa_J37032 BBa_J37032]:plux-GFP(high copy)
4=[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]:ptet-luxR-plux-GFP(low copy)
5=ptet-mLuxR(too sensitive)-plux-GFP
6=N.C
7=ptet-luxR-plux-GFP-plac-aiiA
discussion
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
 "
"