Template:Team:UC Berkeley/Notebook/MJV notes
From 2008.igem.org
Madhvi 05:43, 4 June 2008 (UTC)
Yesterday we talked about biobricks and cloning. Today we talked about assembly, BBb standard, and documentation. We also discussed our project plan today. In our demos, we run a PCR yesterday to amplify GFP from a plasmid. Today, we did a zymo cleanup, a restriction digest, and ran an analytical gel (E-gel).
I set up my notebook today and I am looking at papers to try to find a good choice of protein for the protein purification part of the project. I am also trying to find growth-phase dependent promoters.
Madhvi 05:08, 12 June 2008 (UTC)
Last week, I didn't really find anything regarding a good choice of protein, but I did find the rrnB P1 promoter that robustly turns off when cells enter the stationary phase. I also designed the oligos for my basic parts (variations of Cre, ligase, BglII, BamHI and the rrnB P1 promoter). Construction files for these parts (K112100-K112121) are included in the construction files section of my notebook. All of my oligos have not yet come in. They should be in tomorrow and I should be able to run PCRs tomorrow.
In the meantime, I have been doing a few things to determine the assay conditions for the Gateway reactions. On Friday and Monday, Jin and I made concentrated stocks for the six parts of the Gateway buffer (1M Tris pH 7.4, 5M NaCl, 1M Spermidine, 0.5M EDTA pH 8.1, 50mM DTT, 10 mg/mL BSA). Yesterday, I mixed appropriate volumes of them to make 10mL of a 1x Gateway Buffer (22mM Tris, 100mM NaCl, 7mM Spermidine, 5mM EDTA, 2mM DTT, 1mg/mL BSA) and stored it at 4 degrees. Jin says it should be stable for approximately one week.
Today, Jin and I ran the regular clonase reaction with pBjh1600 (RFP entry vector with p15a origin) and pBjh1601CK (CK assembly vector with p15a origin). The reaction is set up with 0.6uL EB Buffer (Dilute Tris), 0.3uL Assembly vector miniprep, 0.3uL Entry vector miniprep, and 0.3uL LR Clonase II. We also ran this reaction (usually 1.5uL) scaled up to 10uL by adding 8.5uL of 1x gateway buffer. We transformed both products into DH10B and plated on KC plates after 1hr. reaction time. We will compare the number of colonies on both plates tomorrow.
I also am growing up cultures two cultures which we will use tomorrow for a Gateway reaction after arabinose induced lysis. The first culture is TG1 (ccdB immune strand) with pSB3C6-Bjh1311 (holin, antiholin and lysozyme under Pbad promoter; p15a origin), pHA57 (xis, int; high copy number, temp. sensitive origin of replication), and pBjh1600. The second culture is TG1 with pSB3C6-Bjh1311, pHA57, and pBjh1601AK. We will mix given amounts of the two culture, wash and resuspend in our 1x gateway buffer and then lyse with arabinose. We will transform the resulting reaction mixture into DH10B and if the reaction was successful we should see colonies on AK plates.
I am also growing up cultures of Righty and Lefty cells to make competent cells that I can use to make my BamHI and BglII basic parts.
Madhvi 08:02, 17 June 2008 (UTC)
Thursday, I made Righty and Lefty competent cells. When I checked the plates from the Gateway reactions that we ran, I saw only a couple of colonies on the plate where the reaction was scaled up and a lot of colonies on the other plate. So, it looks like the reaction does not really work when scaled up to 10uL. Chris thinks that we need to find more information about the appropriate buffer solution and we need to screen different potential buffer solutions to find one that works.
My oligos also came in on Thursday, so I started running PCRs. Because we couldn't find the templates for the BglII, Cre and BamHI experiments, I ran the PCRs on MG1655 and T4 genomic DNA to clone the rrnB P1 promoter and parts of ligase (used program 55). However, none of the reactions worked. So, on Friday, I tried running the MG1655 PCR with & without DMSO as well as the ligase PCRs and the BamHI PCRs without DMSO (on program 55). The only thing that worked was the MG1655 PCR with DMSO. On Friday, I also transformed appropriate templates into Righty and Lefty cells [pBca9145-Bca1010 (Cre) into Righty, pBca9145-I716210 (BamHI) into Righty and BglII with a promoter into Lefty]. I also did test transformations of BamHI and BglII into the Righty and Leffty competent cells that I made in order to confirm that they were correct (which they were).
On Saturday, I grew up colonies from the plates that I made in order to be able to isolate template. However, the Amp media that I used appeared to be lethal, so I did not have cultures on Sunday. I grew up cultures again on Sunday in new Amp media and was able to miniprep and run PCRs on the Cre and BglII templates today.
In the meantime, did PCRs on the BamHI and ligase parts on Saturday with DMSO at 55. I succeeded in amplifying the first three segments of ligase and in getting products for {r.BamHI!}, {r.BamHI>}, and {<r.BamHI>}. Since the bands for the BamHI parts on the gel were quite faint, I used them as templates on Sunday to amplify them more (I ran these reactions at 55 with DMSO). I also did a SOEing reaction with the first two segments of ligase at 55 with DMSO. I tried to clone the {a~r.BamHI!} and {rbs.r.BamHI!} parts using {r.BamHI!} as a template by running these reactions with & without DMSO at 45. I also tried cloning the 4th fragment on ligase with and without DMSO at 45. I found the the {r.BamHI!} template was impure so the resulting reactions had a lot of background and the products were difficult to resolve on the gel. However, the {r.BamHI>} and {<r.BamHI>} reactions looked good and gave well-defined bright bands. Furthermore, the 4th segment of ligase was successfully amplified both with and without DMSO. The SOEing reaction with the first two segments of ligase was also successful.
Today, I reran the {r.BamHI!} (55 w/ DMSO), {a~r.BamHI!}, and {rbs.r.BamHI!} (both 45 with and without DMSO) PCRs using {r.BamHI>} as a template. I also ran the SOEing reaction with the third and fourth segments of ligase with DMSO at 55). I also ran all of the BglII and Cre reactions with the templates that I miniprepped from my cultures.
Madhvi 03:02, 19 June 2008 (UTC)
All of the PCRs worked except for the SOEing reaction of the third and fourth segments of ligase. So, I went ahead with the next cloning steps (digestion, ligation, and transformation) yesterday. Today, I got colonies on all of my plates except the {r.BamHI>} plate. I will redo the ligation & transformation for this one sample tomorrow. I grew up colonies from all of the other plates today, so that I can miniprep and send them in for sequencing tomorrow. Since the SOEing reaction for the third and fourth segments of ligase didn't work yesterday, I reamplified the third region of ligase and segment E (combination of first and second regions) in a PCR that Christie set up for me yesterday evening. I used the reamplified part C to run SOEing reactions of C & D at both 45 & 55 with and without DMSO. The SOEing reaction worked at 55 without DMSO, so I used the resulting fragment (F) as a template along with fragment E to run the final SOEing reaction for ligase. I will see how it works tomorrow!
Madhvi 02:12, 20 June 2008 (UTC)
The ligase PCR worked with 2K45 w/o DMSO! yay! So, now that gave me the {rbs.ligase!} part. I digested that today and ligated it with the new stock of the digested parent vector that Molly, Aron, and Bing made and purified. I also ligated {r.BamHI>} because that plate had no colonies on it yesterday. I transformed both of the samples into Righty cells and plated. Bing miniprepped all of the samples that I grew up yesterday and sent them out for sequencing.
Jin found another paper that described a slightly different buffer mix for the gateway reaction. We will test it tomorrow using the two strains that have the arabinose induced lysis device, along with an entry/assembly vector, and the vector to make xis and int (TG1 with pSB3C6-Bjh1311, pHA57, pBjh1600 and TG1 with pSB3C6-Bjh1311, pHA57, pBjh1601AK). I grew up these two strains in ACSpec and ACK media, respectively.
Today, Chris presented to us about Adobe Illustrator and I met with Susanna Spiro (from COE), Marlee, and Nade about the blog today. We should be starting that next week.
Madhvi 08:10, 23 June 2008 (UTC)
Friday was a long and eventful day. I got about 20-25 colonies on the {rbs.ligase!} plate and 8 colonies on the {r.BamHI!} plate. Chris helped me run a colony PCR on 8 of the ligase colonies to screen them and make sure that the part that they had was the right size. Basically, each colony was picked swirled around in the appropriate PCR tube then put into a well with LB on a 24 well block. The PCR was run with the standard forward and reverse oligos for BBb Biobrick parts (ca998 and g00101). Out of the 8 clones screened, clone #2 had the wrong part (it was ~1000bp instead of ~1500bp). All of the other clones appeared to be correct on the gel that was run.
I also got back sequencing results. All five of the BglII parts were perfect. All five of the Cre parts were perfect partials--I told Jin to ask them to sequence all five of them with the standard reverse oligo (g00101) so that I can check the last 300bp of each of the Cre parts. The rrnB P1 promoter had a perfect sequence. Out of the four BamHI parts that were sequenced, two of them ({r.BamHI!} and {rbs.r.BamHI!}) were bad reads. {a~r.BamHI!} had a huge deletion in the middle of the part. {<r.BamHI>} had a point mutation, which was not silent. I re-picked colonies for all of the BamHI parts on Friday so that they could be miniprepped and sent for sequencing on Saturday. Jin suspected that the BamHI template that I used (pBca9145-Bjh0029) was bad, so I also set up PCRs using pBca9145-I716210 as template for all of the BamHI parts. I went ahead and set them up at 55 w/ & w/o DMSO and at 45 w/ & w/o DMSO.
On Friday, Jin and I also screened 15 buffers using the cultures that I grew up on Thursday for the Gateway reaction with arabinose-induced lysis. Christie used the stock solutions to make 10mL of the new variation of 1x Gateway buffer (25mM Tris, 6mM Spermidine, 5mM EDTA, 82mM NaCl, 0.5 mg/mL BSA, 2.5mM DTT). The buffers that we screened were: the 2 variations of the Gateway buffer that we had made, NEB1, NEB2, NEB3, NEB4, Expand 2, Expand 3, Phusion GC, Phusion HF, & Fast Digest. Chris had screened many of these by scaling up the clonase reaction, but he got inconclusive results (many of them had both red and white colonies) because the clonase that we have has probably gone bad.
The protocol that we used for the experiment involved doing the following for each sample:
- Mix 300uL of each culture in Eppendorf
- Centrifuge using maximum speed for 30 sec
- Discard supernatent & remove residual liquid using pipette
- Resuspend in 100uL of 1x buffer
- Centrifuge using maximum speed for 30 sec
- Discard supernatent & remove residual liquid using pipette
- Resuspend in 100uL of 1x buffer
- Add 1uL of 20% arabinose
- Incubate at 37 degrees for 1 hour
- Incubate for 1 hour at room temperature
- Centrifuge using maximum speed for 30 sec
- Transform 10uL of the supernatent into 45uL of Righty cells
- Rescue with 50uL of 2YT and incubate for 1 hour at 37 degrees
- Plate on AKG plates (Righty cells have Gentamycin resistance)
After incubating 1 hour at 37 degrees with arabinose, the samples in Expand 3, Gateway Buffer #1 (the one I made originally) and Phusion HF showed apparent lysis (they were viscous when tested with a pipette). Anyway, none of the plates had colonies on them on Saturday. Jin feels that there might be something wrong with the expression of xis and int from the cells, which would explain why none of the reactions worked.
On Saturday, I found that the PCRs for the BamHI parts all worked at 55 w/o DMSO. So, I went ahead and digested, ligated, and transformed all of them BamHI parts into Righty cells. Jin and I also miniprepped the cultures that were grown up on Friday (including my ligase cultures, my BamHI cultures, and some cultures for Dirk and Bing). I also grew up colonies for Aron and Molly from their plates.
Today, I found out that 2 out of the five BamHI parts ({r.BamHI>} and {rbs.r.BamHI!}) that I plated had no colonies and {a~r.BamHI!} had only one colony). Jin thinks that the vector digest that I used was too dilute. I will redo the ligation and transformation steps for those parts tomorrow. I also got sequencing back from yesterday's samples. Ligase #5 is perfect! yay! So, I went in and set up PCRs for the rest of the ligase parts (the one that I have right now if {rbs.ligase!}). I set them up at 2K55 w/ & w/o DMSO and at 2K45 w/ & w/o DMSO. With the exception of {<r.BamHI>}, the other BamIi parts seem to be problematic (either mutations or bad reads again). So, I guess that I will follow through with the cloning from I716210 for the other BamHI parts and see how it goes. I will check the reverse sequences for the Cre parts tomorrow.
Madhvi 06:00, 26 June 2008 (UTC)
On Monday, I analyzed the reverse reads of my Cre sequences. Two of them were bad reads, two of them appeared to be the wrong parts and one of them was correct. Jin sent the four that appeared to be bad for resequencing on Monday and they all were correct in the reads that I got on Tuesday. So, all of the Cre parts that I have made thus far are correct.
On Monday, I also PCRed the {<r.BglII!} and {Cre!} parts because we realized that templates that we had used to clone them were not in Biobricks, so an Eco/Bam transfer would not work for these. I digested the PCR products of this. I also digested the five ligase parts that I PCRed from the {rbs.ligase!} part (all of the PCRs worked well at 55 w/o DMSO). Bing did the ligations and transformations for these ligase parts, the {Cre!} part, the {<r.BglII!} part and for the three BamHI parts that had no colonies or only one colony on the plate on Sunday ({r.BamHI>}, {a~r.BamHI1}, and {rbs.r.BamHI!}). I forgot to tell him to transform the BamHI parts and the into Righty cells and the BglII part into Lefty cells, so he put them all in DH10B. The {<r.BglII!} part works just fine in DH10B cells. He got the labeling for the BamHI plates mixed up, so I am not sure which one is which. Two of the plates had a few colonies and the other one had no colonies. I suspect that the one with no colonies is the {rbs.r.BamHI!} part.
Yesterday, Bing grew up samples from all of the plates from Monday (the 5 ligase parts, the {Cre!} part, the {<r.BglII!} part, and the two BamHI plates that had colonies). He and Molly miniprepped them roday and sent them out for sequencing. I also redid the ligations and transformations of the three BamHI parts ({r.BamHI>}, {a~r.BamHI1}, and {rbs.r.BamHI!})into Righty cells yesterday. However, today I found that these plates had very few colonies (a total of 13 for all three plates). I did colony PCR on 11 of the colonies today and found that only one of them appeared to be the right size. Tomorrow, I will miniprep and send this colony for sequencing; however, I suspect that it is probably not right. Jin thinks that there might have been an accidental tube switch in the process of making these competent cells and these cells might actually be Lefty cells. Today, I asked Sherine and Cici to redo the ligation and transformation of the three BamHI parts (this time I made sure that they transformed into Righty cells).
Yesterday, I also transformed minipreps of all of the BglII parts that I have, the {<r.BamHI>} part, the rrnB P1 promoter, and the {rbs.ligase!} part into DH10B (because they were all made in Lefty or Righty cells and had the BamHI or BglII sites methylated). The BglII/BamHI samples that can survive in DH10B are {<r.BamHI>}, {r.BglII>}, and {<r.BglII>}. Today, I picked colonies from these plates and the rrnB and ligase plates to be miniprepped tomorrow so that we can have plasmids with free BglII and BamHI sites for assembly. From Monday, we also know that the {r.BglII!} part is fine in DH10B. All of them gave hundreds of colonies with the exception of {r.BglII>}, which seems relatively toxic compared to the other three parts.
With regards to Gateway stuff, Chris told Jin to order a small size of Clonase so that we can repeat the experiment where we scale up the reaction with different buffers. We also are planning to do a couple of other experiments. The possible explanations for the failure of Friday's experiment are that the xis and int did not have enough time to catalyze a significant number of transfers (we only left them 1hr to do Gateway, which works for clonase, but maybe the xis and int are being produced at too low concentrations for it to work in 1hr). It also may be a case where they are not catalyzing enough transfers for them to be assayable (the pAH57 plasmid has been used before to park things in the genome via recombination, but this requires only a single transfer). So, we are going to see whether there is an assayable amount of transfers that occur in the cytoplasm over a longer time scale. We are planning to put pAH57 (xis & int cassette; temp. sensitive origin; AmpR), pBca1256 (entry vector; pUC origin; SpecR), and pBjh1601KC (assembly vector; p15a origin; KanR & CamR) into TG1. After growing overnight, we will then miniprep the mixture of plasmids and transform into DH10B and plate on KC plates.
Chris also suggested testing the effect of the xis/int construct on the growth rate of TG1 (they are somewhat toxic, so they have shown slow growth in the past). We are making saturated cultures of the following things and then doing the same dilution on all of them and testing OD after a set amount of time:
- TG1
- TG1 with pAH57
- TG1 with pAH57 & pBca1256
- TG1 with pAH57 & pBjh1601KC
- TG1 with pAh57, pBca1256, & pBjh1601KC
Chris thinks that the toxicity effect might be greater if there are substrates of the xis and int present in the cytoplasm of the cell, but we'll see what happens when we actually do the experiment.
For the Gateway stuff, I transformed pAH57 into TG1 competent cells and plated on Amp plates. Today, I grew up colonies and I transformed either pBca1256 or pBjh1601KC into them when they were at midlog using the protocol posted for transforming into cultures.
Madhvi 08:57, 27 June 2008 (UTC)
The transformation of the BamHI parts from yesterday did not really work again--there was only one colony between all of the three plates. Chris suspects that the toxicity of these parts is leading to problems with the transformation. Today, I did an EcoRI/BamHI digest of the pBjh1600 plasmid (entry vector with p15a origin) and gel purified. I did new PCRs of all of the BamHI parts except {<r.BamHI>}, which sequenced correctly this weekend. I digested and ligated these parts with pBjh1600. Hopefully, moving these parts from a pUC plasmid to a p15a plasmid will reduce the toxicity by lowering the copy number.
I asked Sherine and Cici to miniprep the DH10B colonies that I grew up yesterday. They also miniprepped the one BamHI colony that looked right in the colony PCR and I sent it out for sequencing today.
Since Sam's BamHI plasmid, pBca9145-I716210, was miniprepped from Righty earlier, I asked Molly to transform it into DH10B. She also transformed pBjh1600 into DH10B so that we can miniprep and replenish Jin's stock that I used today.
The transformations that I did yesterday of pBca1256 & pBjh1601KC into TG1 with pAH57 did not work well because I did the rescue at 37 degrees instead of 30 degrees. I diluted a saturated culture from yesterday and grew it to midlog & redid the transformation.
I looked at the sequencing data that I got today.
Madhvi 00:53, 29 June 2008 (UTC)
For the gateway experiment, I did the following transformations:
- pBjh1601KC into TG1 with pAH57 and pBca1256
- pAH57 into TG1 with pBca1256
- pBjh1601KC into TG1 with pBca1256
- pAH57 into TG1 with pBjh1601KC
Madhvi 23:13, 29 June 2008 (UTC)
- miniprep K112103 from DH10B
- put correct plasmids in stock plate
- colony PCR of BamHI parts
- transformations for Gateway
Madhvi 01:03, 1 July 2008 (UTC)
- pBca1256 into TG1 with pBjh1601KC and pAH57
- pBjh1601KC into TG1 with pBca1256 and pAH57
- pAH57 into TG1 with pBca1256 and pBjh1601KC
Madhvi 00:06, 2 July 2008 (UTC)
Today, my sequences for the BamHI ({r.BamHI>}, {a~r.BamHI!}, and {rbs.r.BamHI!}) came in and at least one of each clone sequenced correctly! So, I guess the trick was putting them in a p15a plasmid after all. So now I am officially done with basic parts!
Since the colony PCRs from both yesterday and Sunday did not give good results (because the thermocycler was set to do 10 repetitions instead of 30), I set up another colony PCR with the cultures that sequenced correctly in order to verify that the procedure works with the correct cycle. Indeed, the gel looked good with nice bright bands around 700bp. Since the big thermocyler (the one that is broken right now) is made by the same company, it should work just fine if we run the same cycle on it.
I am also doing a buffer screen with the new LR Clonase kit that we got. This kit has the buffer separate from the enzyme, so we can truly test the effect of the different buffers without changing the volume. The protocol that we followed is:
- Add the following to a 0.5mL tub at room temperature and mix:
- Entry Plasmid (pBjh1600): 0.33uL
- Assembly Plasmid (pBjh1601KA): 0.33uL
- 5X Buffer (LR Clonase, Gateway Buffer #1, Gateway Buffer #2,Phusion HF): 0.66uL (For Expand Buffer 3 that is 10X, add 0.33uL Buffer and 0.33uL water)
- TE Buffer, pH 8.0: 1.5uL
- Remove the LR Clonase enzyme mix from the -80C freezer and thaw on ice for about 2 minutes. Vortex the LR Clonase enzyme mix briefly twice (2 seconds each time).
- Add 0.66uL of Clonase enzyme mix to the reaction and mix well by vortexing briefly twice. Microcentrifuge briefly.
- Return LR Clonase enzyme mix to -80C storage immediately after use.
- Incubate reactions at room temperature for 1 hour.
- Add 0.33uL of Preteinase K solution to each sample to terminate reaction. Vortex briefly. Incubate samples at 37C for 10 minutes. Let DH10B competent cells thaw on ice during this incubation period.
- Add 30uL KCM and 50uL water to the competent cells. Aliquot 50uL of this mixture into each Gateway reaction mixture on ice. Remember to do a negative control (just plain DH10B cells).
- Leave on ice for 10 minutes then heat shock at 42C for 90 seconds. Put tubes back on ice for 2 minutes.
- Rescue with 100uL of 2YT and incubate in the 37C shaker for 1 hour.
- Plate of Amp/Kan plates.
The buffers that we are screening (Phusion HF, Expand 3, Gateway #1, Gateway #2) are the ones that showed apparent lysis (were viscous after 1 hour incubation with arabinose) when we did our previous experiment as well as both of the Gateway buffers we made based on publications that we found. Since this experiment called for 5x buffer, I mixed 1mL of each buffer at 5x concentration. Using our
Madhvi 04:59, 10 July 2008 (UTC)
Yesterday:
- Shirene & I recorded the screening results and sis a colony PCR on the things that were picked the day before
- Since our google spreadsheet making was getting out of control, we discussed how to streamline it with Terry. We decided to fuse "digestion map analysis" and "screening & colony PCRs for transfers."
- Christie worked on updating the "screening & colony PCRs for transfers" so that we have the expected band lengths for all of the parts when we do restriction digests. She also made sure that enzymes that we use will give bands that don't overlap & give big enough bands (>300 bp).
Today:
- minimeeting
- everyone miniprepped
- Dirk & I - zymo yesterday's minipreps
- digestion map with 3uL of zymo worked
- Mach 1 and DH10B comp cells that we made on Tuesday are contaminated--grew up new cultures and streaked cultures on different antibiotic strains to make sure that the strain is ok
to do:
- put results of digestion mapping online
- make tss
- put colony PCR results on google doc
- BamHI/XhoI digestion map of small set of this morning's minipreps & questionable things
Madhvi 23:00, 13 July 2008 (UTC)
Protocol for today's gateway experiment:
- Grow up cultures of Mach1 with pSB3C6-pBjh1311 to saturation
- Dilute approximately 100X (50uL culture in 5mL media) and grow to mid-log
- Spin down 600uL of culture in each 1.5mL eppendorf. In Friday's experiment, I spun down 750uL of culture in each tube
- Wash with 500uL of 1X buffer
- Estimate the volume of the cell pellet and add the appropriate amount of 5X buffer
- Add 1uL of 20% arabinose to each tube and incubate at 37C for 1 hour
- Use 0.66uL of these mixtures in place of 0.66uL 5X buffer when setting up gateway reactions (0.33uL entry vector (pBca1256), 0.33uL assembly vector (pBjh1601KC), 1.32uL water, and 0.66uL clonase enzyme)
- Pipette up and down and vortex to mix reagents
- Incubate at room temperature for 1 hour
- Terminate the reaction by adding 0.33uL of proteinase K and incubate at 37C for 10 minutes
- Transform into 50uL of DH10B competent cell mixture (combination of 220uL cells, 30uL KCm, and 50uL water)
- Rescue and plate on appropriate double antibiotic plate (since the assembly vector that I am using is KC (found in well A7 of Jin's stock plate), I plated on KC plates)
Madhvi 19:41, 16 July 2008 (UTC)
To do today:
- miniprep ig1122 and ig1096
- do transfers using these minipreps
- digestion map these minipreps
- transform entry and assembly vectors into TG1 cells with Bjh1311 (pBca1254AK and pBca1256 into TG1 with pSB3C6-pBjh1311; pBjh1600 and pBjh1601KC into TG1 with pSB1A2-pBjh1311)
- grow up colonies from assembly plates (streak on plates for screening)
- spreadsheet of assembly parts
- grow up DH10B and Mach1 for comp cells
- analyze digestion map gel from yesterday
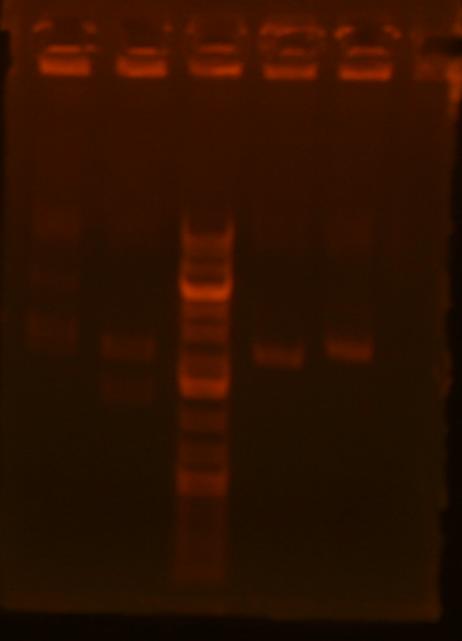 Lane 1: ig1096 (clone#1) - BamHI/XhoI;
Lane 2: ig1122 - BamHI/XhoI;
Lane 4: pKD3 miniprep undigested;
Lane 5: pKD4 miniprep undigested
Lane 1: ig1096 (clone#1) - BamHI/XhoI;
Lane 2: ig1122 - BamHI/XhoI;
Lane 4: pKD3 miniprep undigested;
Lane 5: pKD4 miniprep undigested
Madhvi 16:01, 17 July 2008 (UTC)
to do:
- analyze gels from the past two days
- talk to Chris about finding another conditional origin of replication
- miniprep & digestion map the samples grown up yesterday
- transfer {b1006} to [A/C]
- grow up cultures from yesterday's transfers and assemblies
- grow up cultures for the other ig parts needed later in assembly
- make DH10B and Mach1 comp cells
- grow up cultures of the TG1 cells with lysis plasmid and assembly/entry plasmid for gateway expt
Madhvi 20:50, 18 July 2008 (UTC)
- analyze gels and put on wiki
- gateway expt
- Mach 1 comp cells
- miniprep & digestion map cultures from yesterday
- 3 assemblies & 2 transfers
Madhvi 22:32, 22 July 2008 (UTC)
- redo transfer of <ligase! because the sequence of mv16 showed that the part I used was actually rbs.ligase!
- miniprep, digestion map and send mv11 for sequencing
- send mv10 for sequencing
- gateway
- grow up cultures of mv4, mv7, and mv15
- cotransform pkd3 and pkd4
- test righty and lefty cells
Madhvi 20:21, 24 July 2008 (UTC)
- update sequencing log
- run gel on digestion maps of mv4, mv11, and mv15
- send out samples that digestion map correctly for sequencing
- regrow a colony for the mv1 transfer ({rbs_BglII!})
- grow up colonies of ig1094 and ig1098 from yesterday's plates
- miniprep & digestion map <ligase! cultures from yesterday
- throw away anything that has lamB and phoApp to avoid future confusion because they are wrong
- remind richard to reverse sequence mv11
- send more of MJV076 (ig114-1) for sequencing
- set up PCR for rbs.TrfA and write up construction file
Madhvi 21:34, 25 July 2008 (UTC)
- run gel for digestion map of ig1090 samples
- send one out for sequencing
- miniprep {rbs_BglII!} culture and redo mv1 transfer
- miniprep & digestion map ig1094 and ig1098 samples
- transform ig1124-1 into Righty
- email Richard about reverse sequencing for mv11
- send a different sample of pBad for sequencing
Madhvi 18:51, 26 July 2008 (UTC)
- update sequencing log
- run gel for samples of ig1094 and ig1098
- send ig1094 and ig1098 for sequencing
- pick colonies for mv1 and ig1124
- assembly of mv7
- write construction file for rbs.TrfA & get sequence from Jin for parts spreadsheet
- email Richard about reverse sequencing for mv11--give him more DNA
 "
"