|
← Yesterday ↓ Calendar ↑Tomorrow →
For Kok-Phen
I did some digestions(today), ligations(tomorrow) and screening (the day after tomorrow) for you.
I tried to build :
RBS (B0034) + OmpR*
and
RBS (B0034) + EnvZ*
Protocol
| Digestion name
| Template DNA
| Enzymes
| Volume of DNA
|
| D 158
| MP 155.1 - OmpR*
| XbaI - PstI
| 5 µL
|
| D 159
| MP 156.1 - EnvZ*
| XbaI - PstI
| 5 µL
|
| D 102
| MP 100 - B0034
| SpeI - PstI
| 5 µL
|
- X µL of Template DNA
- Buffer (n°2) 10X : 3µL
- BSA 100X : 0.3µL
- Pure water qsp 30 µL
- 1 µL of each enzyme
- Incubate during about 3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes). Then 10°C overnight.
Transformation of the ligations we did yesterday
We transformed L 143, L 144 and the negative controls T1 and T2, using Invitrogen's TOP10 chemically competent cells standard protocol.
PCR amplification of flhDC and its promoter
List of PCRs
| Name of the PCR
| PCR 136
| PCR 137
| PCR 138
| PCR 136'
| PCR 137'
| PCR 138'
| PCR 139
| PCR 140
|
| Forward primer
| O 110
| O 111
| O 131
| O 110
| O 111
| O 131
| O 110
| O 131
|
| Reverse primer
| O 113
| O 113
| O 132
| O 113
| O 113
| O 132
| O 113
| O 131
|
| Template DNA
| MG 1655
| MG 1655
| MG 1655
| xx
| xx
| xx
| PCR 130
| PCR 130
|
Protocol
We followed the standard protocol of amplification in Two steps.
PCR program used :
PHUSION2
Results
Settings Gel 1%
- Ladder 1 kb 10 µL
- 4µL Template DNA + 2µL Loading Blue
- 1 % Agar
| PCR Name
| What's in ?
| Well
| Expected size
| Measured size
|
| PCR_138
| gene flhDC
| 2
| 992 bp
| 1000 bp
|
| PCR_140
| gene flhDC
| 3
| 992 bp
| 1000 bp
|
| PCR_138'
| gene flhDC
| 4
| X
| X
|
| PCR_133
| gene flhDC + promoter
| 5
| 1227 pb
| 1200 bp
|
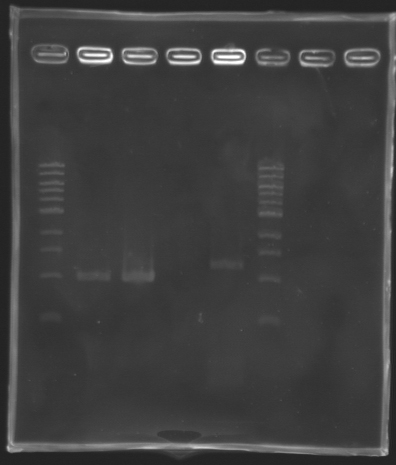
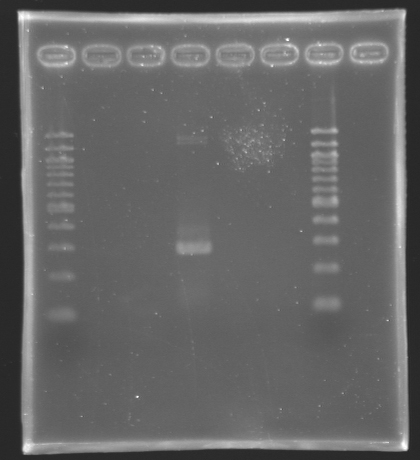
We managed to amplify flhDC !
Settings Gel 2%
- Ladder 100 bp 10 µL
- 4µL Template DNA + 2µL Loading Blue
- 2 % Agar
| PCR Name
| What's in ?
| Well
| Expected size
| Measured size
|
| PCR_136
| pflhDC (URI)
| 2
| 282 bp
| X
|
| PCR_137
| pflhDC
| 3
| 428 bp
| X
|
| PCR_139
| pflhDC (URI)
| 4
| 282 bp
| ~ 300 bp
|
| PCR_136'
| pflhDC (URI)
| 5
| X
| X
|
| PCR_137'
| pflhDC
| 6
| X
| X
|
We managed to amplify the promoter of flhDC
Digestions
After having succeded in amplifying the promoter and the gene of flhDC, we decided to clone it into a plasmid.
The first step is the digestion.
Protocol
| Digestion name
| Template DNA
| Enzymes
| Volume of DNA
|
| D 153
| PCR 138 - g flhDC
| EcoRI - PstI
| 5 µL
|
| D 154
| PCR 140 - g flhDC
| EcoRI - PstI
| 5 µL
|
| D 155
| PCR 139 - p flhDC
| EcoRI - PstI
| 5 µL
|
| D 145
| MP122 - pSB1A2
| EcoRI-SpeI
| 5 µL
|
| D 136
| MP103 - J61002
| EcoRI-SpeI
| 5 µL
|
- X µL of Template DNA
- Buffer (n°2) 10X : 3µL
- BSA 100X : 0.3µL
- Pure water qsp 30 µL
- 1 µL of each enzyme
- Incubate during about 3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes).
Then 10°C overnight.
Digestion
Measurement of concentration of minipreps
standard protocol
| Digestion
| Miniprep used
| Concentration (µg/mL)
| ratio 260/280
|
| D146
| MP148.2
| 123
| 1.58
|
| D147
| MP153.3
| 113
| 1.68
|
Digestion
Protocol Digestion
Ligation
Protocol Ligation
| Ligation name
| Insert
| Vector
|
| L150
| D146 (strongest rbs-TetR-GFP tripart)
| D105 (pLas)
|
| L151
| D147 (strongest rbs-LasR activator with LVA)
| D125 (Double terminator)
|
| Control 1
| /
| D105
|
| Control 2
| /
| D125
|
| Positive Control
| Puc19
|
Analysis of yesterday PCR screening
- 1% agarose gel
- 10 µL loaded
| Gel n°1
| well n°
| 1
| 2
| 3
| 4
| 5
| 6
| 7
| 8
| 9
| 10
| 11
| 12
| 13
| 14
| 15
| 16
| 17
|
| sample
| 1 kb DNA ladder
| positive control 1
E0240
| positive control 2
pSB3K3
| negative control
| L139.1
| L139.2
| L139.3
| L139.4
| L139.5
| L139.6
| L139.7
| L139.8
| 100 bp DNA ladder
| L140.1
| L140.2
| L140.3
| L140.4
|
| expected size
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| measured size
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gel 1 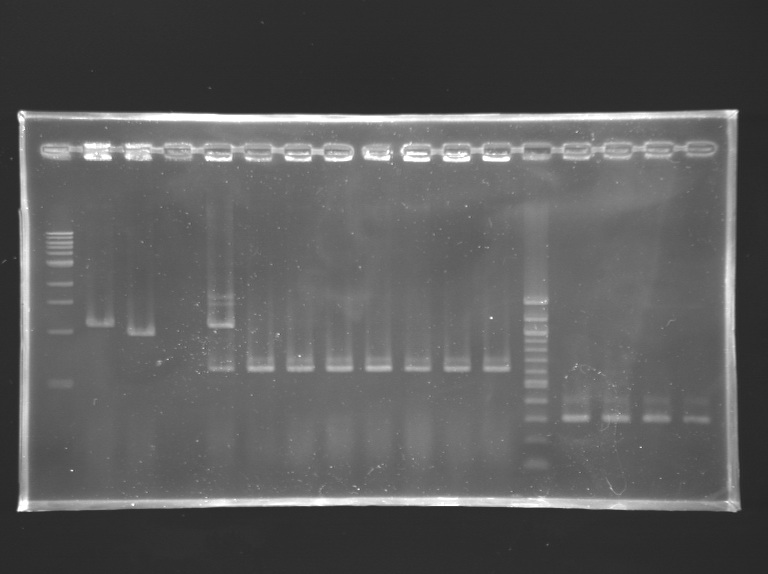 Gel 2
Gel 2 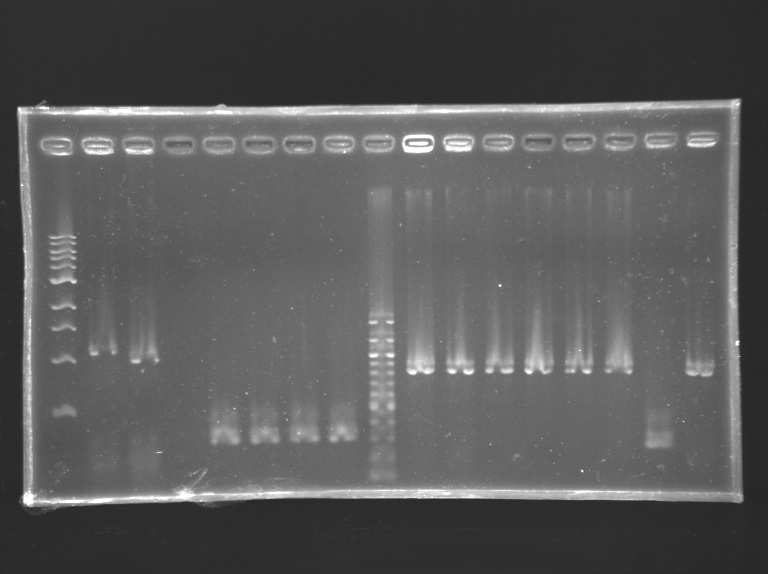 Gel 3
Gel 3 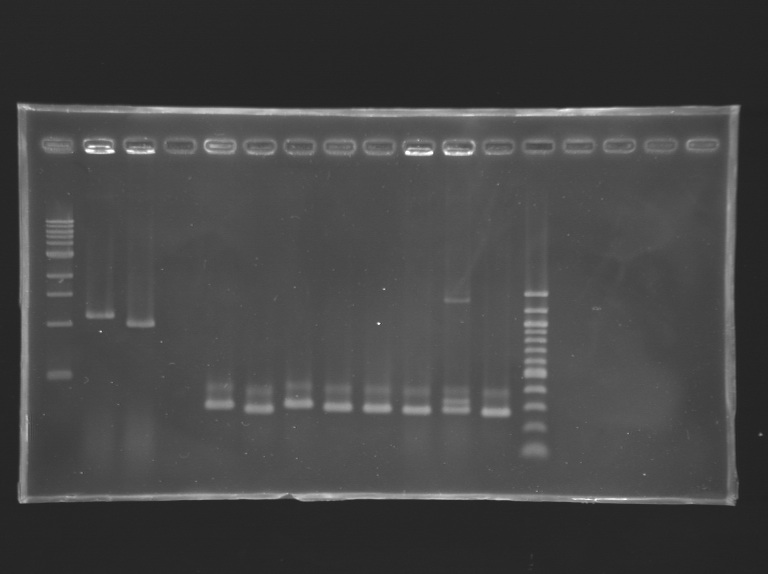
Starting the construction of the Promoter characterization plasmid
Digestion
Measurement of concentration of minipreps
standard protocol
| Plasmid
| Miniprep
| Concentration (µg/mL)
| ratio 260/280
|
| MP3
| 3
| 152
| 1.43
|
| MP3
| 4
| 775
| 1.21
|
| MP101
| 1
| 317
| 1.66
|
| MP101
| 2
| 389
| 1.36
|
| MP101
| 4
| 209
| 1.76
|
| MP104
| 1
| 173
| 1.32
|
| MP104
| 3
| 43
| 1.85
|
| MP104
| 4
| 52
| 1.66
|
| MP114
| 1
| 173
| 1.75
|
| MP114
| 2
| 263
| 1.43
|
| MP143
| 1
| 133
| 1.55
|
| MP143
| 2
| 132
| 1.70
|
Digestion
Protocol Digestion
| Plasmid
| Description
| Miniprep used
| Enzymes
|
| MP3
| B0015 (double terminator B0010-B0012)
| 4
| EcoRI and XbaI
|
| MP114
| TetR
| 1
| EcoRI and SpeI
|
| MP104
| PTet (Tet promoter)
| 1
| SpeI and PstI
|
| MP101
| promoter J23101
| 1
| SpeI and PstI
|
| MP143
| gfp generator
| 2
| SpeI and PstI
|
|
 "
"