Team:Valencia/Project/Lab work
From 2008.igem.org
Contents |
1.- Construction of a Liquid Culture Calorimeter (LCC).
MaterialsTo build our own LCC we need the following material:
|
Method
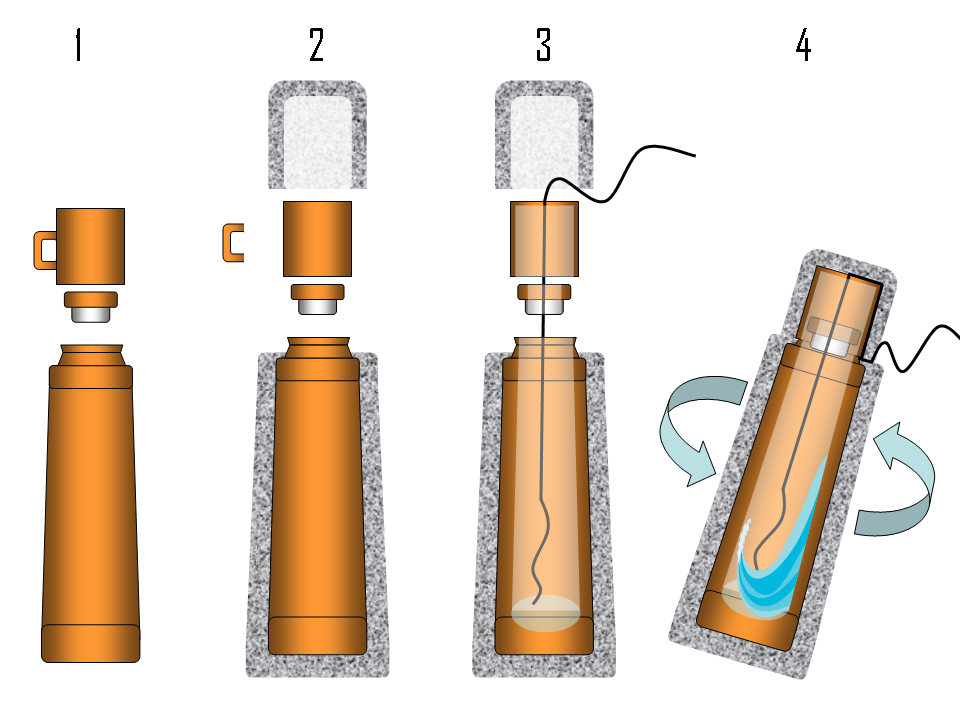
Building your own calorimeter system is easy. You just need to follow the next steps:
- Select the measurement device, in our case a type T thermocouple(Cu-constantan).
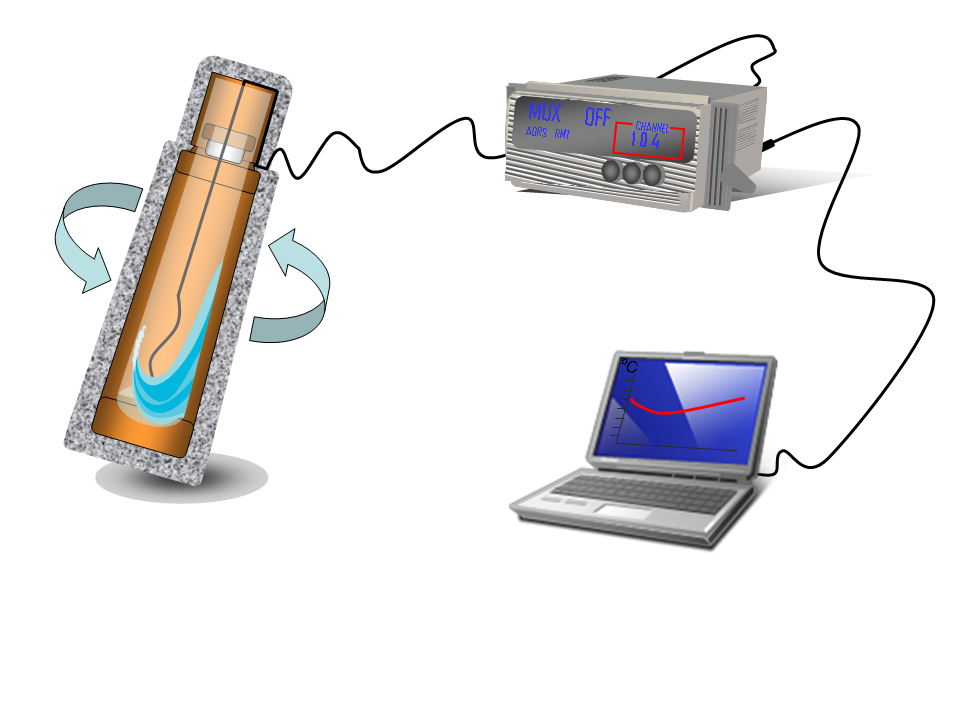
- Build the thermocouples. It is very easy!! You just peel both cables then join them and you obtain a thermometer of high precision. To see the temperature measurements of the thermocouples we connect them to a voltage acquisition card inserted on a data logger.
- Go to a calibration bath and calibrate the thermocouples according to a standard thermoresistence. The precision of the system was up to 0.01 K degrees!
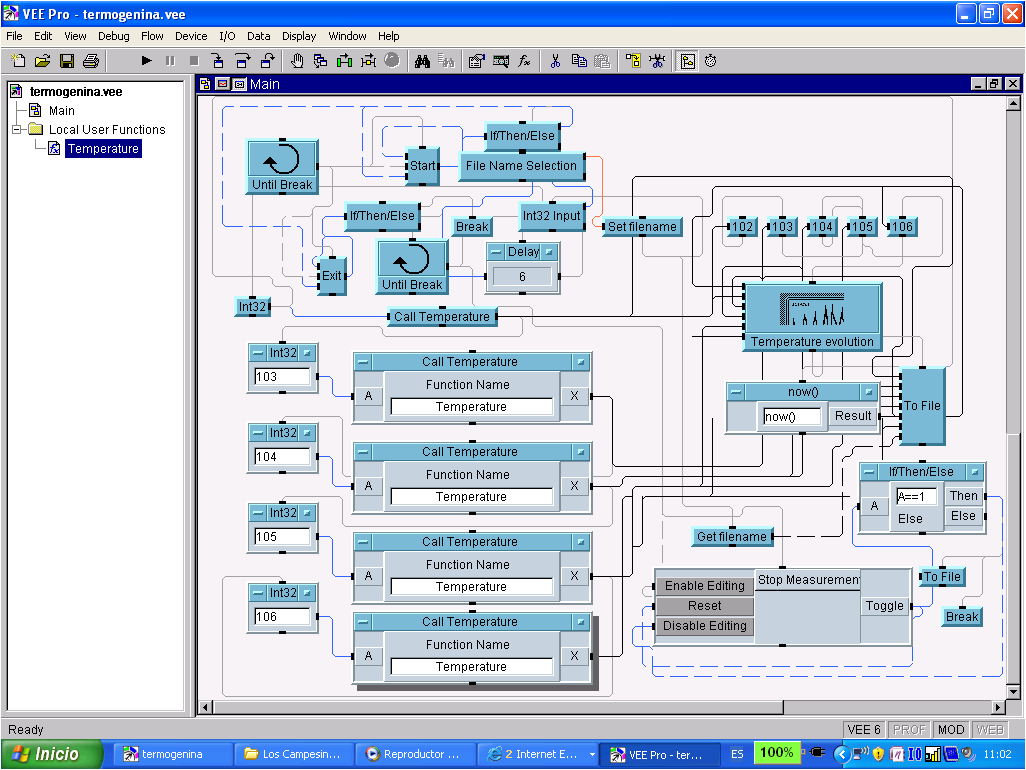
- To complete the temperature measurements acquisition process, an acquisition program using Vee pro was developed so as to allow the communication between the data logger and a computer (a RS-232 cable was used). Trough this program, we can select the time period in which the measurements have to be taken and storage the temperature readings in the computer. We can also see the temperature evolution during the experiments in real time.
- Take a commercial plastic and glass thermo flask (in our case we take four) and proceed to the following modifications:
• Cut off the handle and drilled a hole in the cap. Then, insert the thermocouple through the hole.
• Cover the thermo flask with insulation foam called Armaflex© in order to improve its thermal isolation.
• Introduce a garbage bag between the flask and the Armaflex© in order to prevent this material from getting wet.
| 
|
Characterization results
After trying different combinations of revolution speed, flask tilt and liquid volume, we obtained a combination of conditions suitable for the experiment( allow a proper respiration of yeast culture): 250 rpm, approximately 10 º tilt.
In this conditions, the 4 LCC were characterized with water at the temperature of the future experiments, in order to stablish the range of heat losses to the ambient of the system. The results are shown in the following figures:
Troubleshooting
We have encountered some adversities regarding Armaflex©. This material is sensitive to mechanical damage and especially to water. No matter how careful we were, we found the insulation capacity of our system had drastically decreased in the first weeks.
| The main problem was cleaning the LCCs, since it was very difficult for them not to get wet. We tried different protocols, such as covering them with aluminum foil and sealing with parafilm or using funnels, but the water got inside anyway. Every time the temperatures were falling more than usual, we had to stop the experiment, remove Armaflex© cover and use a hair dryer to dry it.
|
| With regard to the LCCs characterization, we have also gone through different conditions combinations. The first problem we encountered was the apparition of sudden periodic oscillations of temperature in some of the experiments. The best explanation was that water was somehow coming in contact with the thermocouple, which was placed in the upper part of the flask at that time. To try and solve that, we reduced the liquid volume, but the problem persisted. After watching this phenomenon was not produced when the shaking was stopped, it became clear the oscillations were related to it, probably because of a condensation process. |
The solution to this problem appeared, as usual, when we were not looking for it. In order to provide more oxygen to the culture, we increased the shaking speed and the flask tilt in an experiment with water. Surprisingly, the periodic oscillations disappeared and the temperature not only did not fall so much, but slightly increased. We found we could regulate the temperature with these two factors; therefore, we adjusted them so as to obtain almost stable temperature with a high shaking speed suitable for culture growth.
Another condition we changed was the position of the thermocouple inside the flask. They had been always placed in the upper part, having no contact with the liquid because of contamination reasons. However, our engineer team members suggested it would be better for the thermocouples to be submerged in the liquid. After carrying out an experiment, we saw there was not too much different between both positions, but there were not contaminations problems either. Since the data obtained is more exact with the thermocouple submerged, we decided to keep them that way.
 "
"