|
__september
Sep. 10th 2008
CMV PCR (Sabine)
-PCR with primer for the CMV promotor; as DNA template the CMV+RLuc construct from the Ljubljana group (isolated from the parts collection 2007) was used.
For a 50 µl reaction:
40,4 µl H2O
5 µl buffer (10x)
1,5 µl FWD Primer (15pmol)(
1,5 µl REV Primer (15pmol)
1 µl dNTP (10mM)
0,1 µl DNA template
0,5 µl Pfu Polymerase
The settings for the PCR machine are the following:
1. T=94°C 00:02:00
2. T=94°C 00:00:30
3. T=62°C 00:00:30
4. T=72°C 00:01:00
5. GOTO 2 REP 29
6. T=72°C 00:10:00
7. HOLD 6°C
No product was received.
Sep. 11th 2008
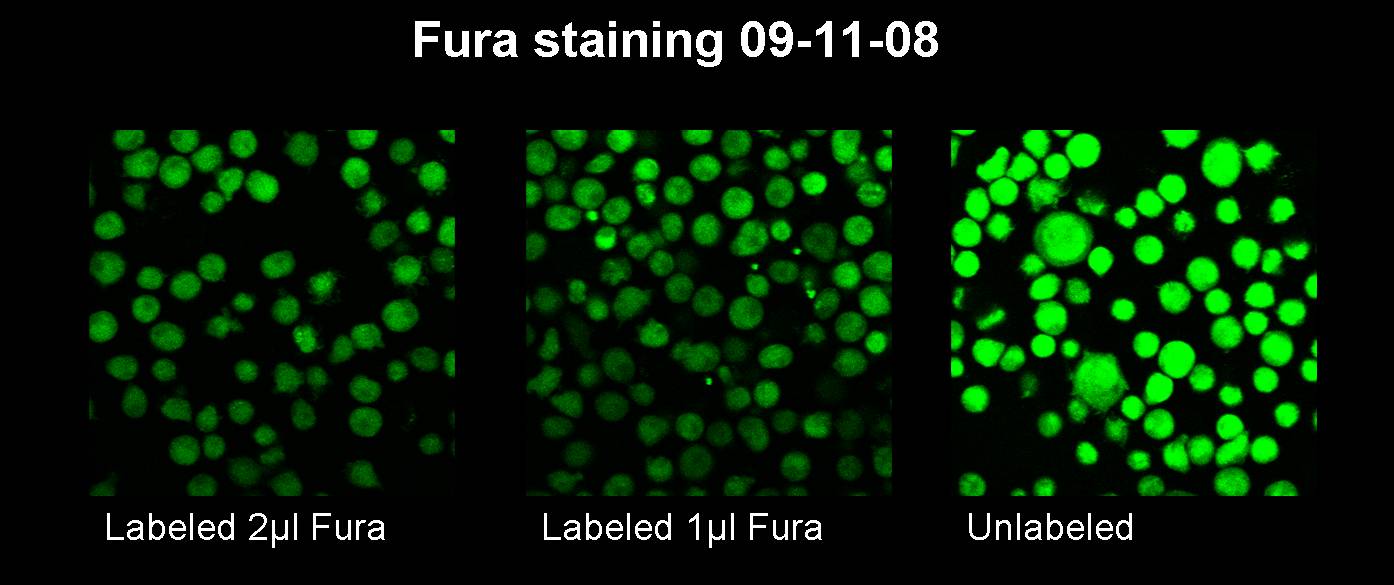
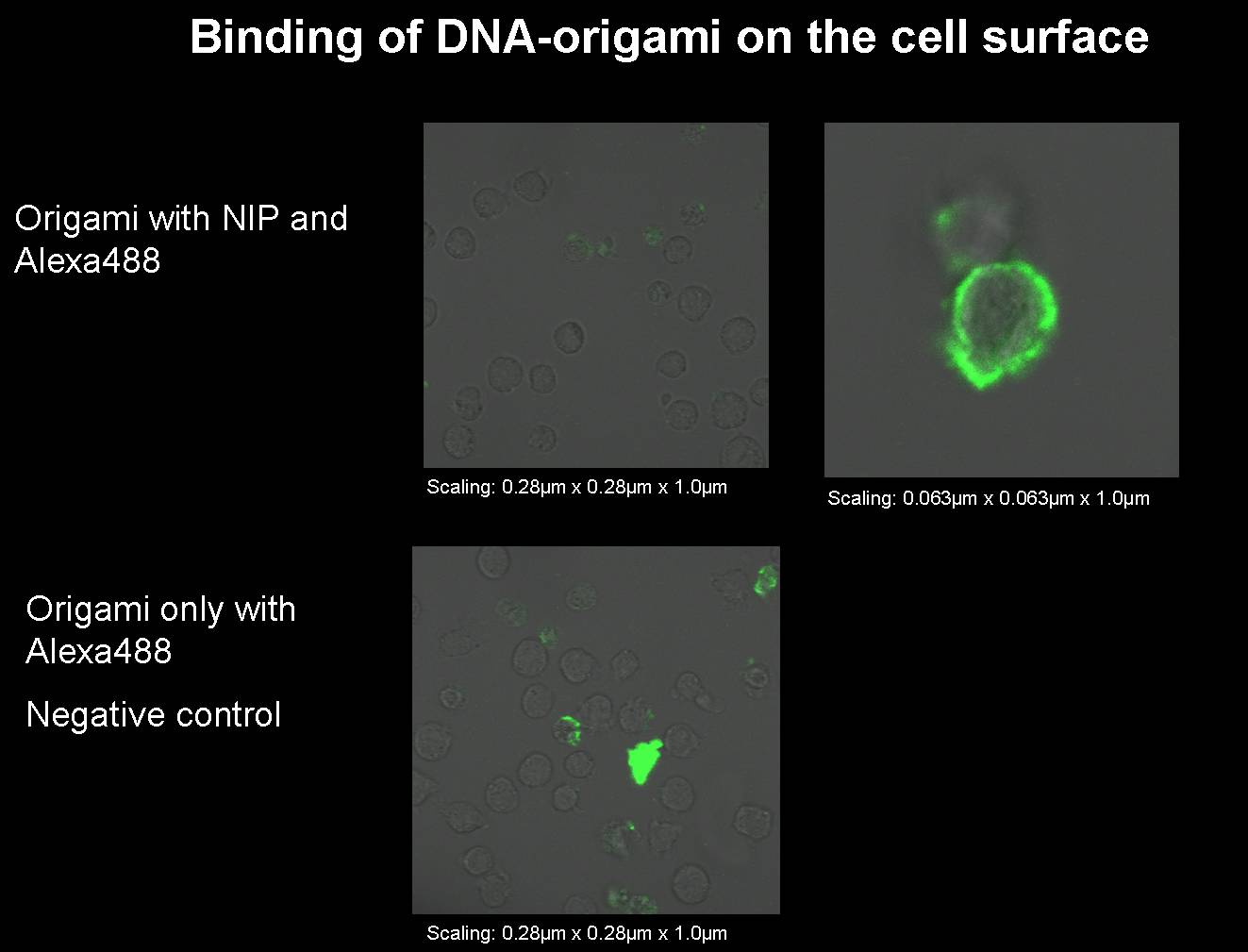
Sep. 12th 2008
1) Origami with NIP and fluorophor for the binding measurement (Norman+Simone)
We had to produce some new origami for our next binding measurements.
- Origami with NIP and fluorophor
- Origami only with fluorophor (without NIP); negative control
see at the protocol from 07-24-2008
2) Origami for the Calciummeasurement (Norman+Simone)
- Origami without NIP (negative control)
see at the protocol from 07-24-2008
To increase the concentration of origami we also made to probes with the double amount
ingredients of the protocol from 07.24.2008
|
Origami with NIP (6x (1:5)) [µl] |
Origami without NIP (6x (1:5)) [µl] |
| Oligos-Pool |
43,68 |
43,68 |
| remainders |
2,4 |
2,4 |
| MgAc |
1 |
1 |
| Phage DNA (448,4 mg/µl) |
33,6 |
33,6 |
| NIP-Oligo |
1,68 |
---- |
| Pool oligo without fluorophor |
0,72 |
0,72 |
| Oligo without NIP |
---- |
1,68 |
3) Master cycler (Norman+Simone)
The origamis were produced in the mastercycler as explained before.
4) Purification of the DNA Origami (Norman+Simone)
Was done as before
5) Digestion of CMV+Rluc (Sabine)
Digestion with EcoRV und FspI (3h at 37°C)
- 5µl plasmid
- 10µl H2O
- 0,5µl enzyme
- 2,5µl buffer (2)
- 0,5µl BSA
File:Verdau CMVRluc EcoRV FspI klein.jpg
6) CMV PCR (Sabine)
-the same PCR with different annealing temperatures using a gradient between 58°C and 62°C (optimal annealing temperature: 62°C)
-again, no products were gained
Sep. 15th 2008
CMV-PCR (Sabine)
-another effort to gain the CMV-Promotor via PCR, this time with different polymerases: Taq Polymerase and a Mix.
-one approach with the complete plasmid and one with the digested plasmid (see digestion of CMV+Rluc)
-products in the approach with taq polymerase as well as in the approach with the Mix. 3 bands from each approach were cut out
Sep. 16th 2008
Gel purification (Sabine)
-gel purification of the PCR products
Digestion of the PCR products and the transfectionvector (Sabine, Kathrin)
-digestion with EcoRI and PstI
-ligation
Sep. 18th 2008
Transformation (Sabine)
-transformation with the ligation product.
Sep. 19th 2008
Transformation (Sabine)
-no colonies on the plates.
Sep. 20th 2008
Digestion of the PCR products and the transfection-vector (Sabine)
-the restriction-enzymes XbaI and SpeI were used
Gel purification and ligation (Sabine)
-digestion of PCR products and vector
Sep. 21st 2008
Transformation of the ligation (Sabine)
-RV 308 cells were transformed with 10 µl of the ligation
Sep. 23rd 2008
1. Transformation of the 4 plasmids of ATG (michael)
-> Signalpeptide
-> Transmembrane domain
-> His-tag
-> Strep-tag
2. Digestion...
Sep. 24th 2008
1. Transformation (michael)
of the Ligations: CMV-Promotor + Vector
proportion: PCR-product (CMV promoter) / vector
-> 6/2
-> 4/4
2. Picking clons of the '4 ATG trafos' (michael)
-> Signalpeptide
-> Transmembrane domain
-> His-tag
-> Strep-tag
after growing glycerinstocks and Miniprep of all 4
3. Picking clons from stock (michael)
-> lipocalin
-> C-GFP and N-GFP
-> C-CFP and N-CFP
-> scFv anti NIP
4. Digestion of the CMV-promoter
Approach Mix: 3/4.1/4.2/4.3/4.4
...
Sep. 25th 2008
Miniprep (normann)
of C-CFP and scFv-anti-NIP
Picking from stocks (normann):
N-CFP, N-GFP, C-CFP, C-GFP
Defrost B12-cells (michael)
and put in new RPMI:
->500ml RPMI
+50ml FCS(10%)
+5ml Hepes(1M)
+5ml L-glutamine(200mM)
+5ml Pen-Strep
+1,75µl ß-Mercaptoethanol(14.3M)
Ligation 2nd try (sabine)
with T4-ligase:
Insert: CMV-Promotor, old and new PCR-Product
Vector: Transfection-Vector fermented with EcoRI/SpeI
-Approaches (for each, old and new PCR-Product): Insert/Vector -> a) 6/2, b) 4/4
Sep. 26th 2008
Transformation of the 'ligation 2nd try' (michael)
Picking clones
4x Signalpeptide
2x Transmembraneregion
Miniprep of: (norman)
-Lipocalin
-Venus split N-GFP
-Venus split C-GFP
-Cerulan split N-CFP
|  "
"


