Team:Illinois/Antibody Receptor Tyrosine Kinase Fusion Notebook
From 2008.igem.org
| Home | Team | Project | Notebook | Research Articles | Parts | Protocols | Pictures |
July 1, 2008
Made Yeast Media
YPD Medium, per liter:
10g yeast extract
20g peptone
20g dextrose
20g agar(only for plates)
1. Dextrose filter sterilized, the rest autoclaved
2. Weigh nutrients into flask double the volume you want to make, and stir to dissolve
3. Dextrose added to autoclaved media to equivalent of 20 g/L
4. Liquid media placed on bench, plate media placed in 65 degree water bath approximately 5 minutes
5. Poured into plates and allowed to solidify
July 17, 2008
E.Coli Media
LB medium, per liter
10g tryptone
5g yeast extract
5g NaCl
1 mL 1N NaOH
15g (agar for plates)
1. Antibody DNA resuspended in TE buffer, 0.1 μg/μL
2. 5mL LB inoculated with single colony DH5a pro
3. Incubated at 37 degrees overnight
July 18, 2008
We made competent cells to use for transformations.
Procedure:
1. 3mL overnight culture of DH5a pro
2. Inoculated into 35mL of LB
3. OD600 checked, want 0.2-0.3
4. Place culture on ice for 3 minutes
5. Spin at 10,000 rpm for 7 minutes, discard supernatant
6. Resuspend in 10 mL cold 30 mM CaCl2, spin as before
7. Resuspend in 3 ml cold 30 mM Cacl2, incubated on ice overnight
7. Glycerol added to 10%, cells in freezer
July 19, 2008
Transformation: We received the synthesized TE33 antibody genes from IDT on plasmids. We transformed these plasmids into DH5a cells and plated on LB+amp to screen for transformants.
1. Cells put on ice 30 min with 1μL plasmid(100ng)
2. Heat Shock for 2 minutes at 42 degrees
3. Ice for 8 minutes
4. Add cells to 1ml LB
5. Grow for 1 hour
6. Plate 100 to 200 μL on LB amp, grow overnight
July 21, 2008
Observations:
There were plenty of transformants on the plates. 5ml of LB+amp was inoculated for plasmid extraction the next day.
July 22, 2008
Mini spin prep kit was used to extract the plasmids carrying the TE33 light and heavy chains. From these plasmids we can run PCRs to amplify the regions of the antibody chains.
DNA eluted in 100μL H2O and frozen.
July 29, 2008
All primers brought to standard concentration, 30mM
33.3μL H2O added per mole of primer
July 30, 2008
Assembly PCR: The antibody genes synthesized by IDT contained the entire antigen binding fragments of the light and heavy chain, but to construct a single chain antibody we only need the variable regions of these genes. This PCR will amplify just those sections of the gene and will leave both the light and heavy chain fragments with complementary ends that can be assembled in a following PCR.
| tube | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Antibody chain | H | H | L | L | H | H | L | L |
| DNA source | mini-prep | synthesized IDT genes | ||||||
| template | 0.5ul | |||||||
| primers | 1ul of appropriate forward and reverse primer | |||||||
| MgCl2 | 3ul | 5ul | 3ul | 5ul | 3ul | 5ul | 3ul | 5ul |
| master mix | 20ul | |||||||
| H20 | to 50ul total volume | |||||||
Master mix already contains MgCl2; should have added extra to some tubes.
PCR program
- 94 degrees 5 min
- 94 degrees 1 min
- 50 degrees 1 min
- 72 degrees 1 min
- GOTO 2. 29 cycles
- HOLD at 4 degrees
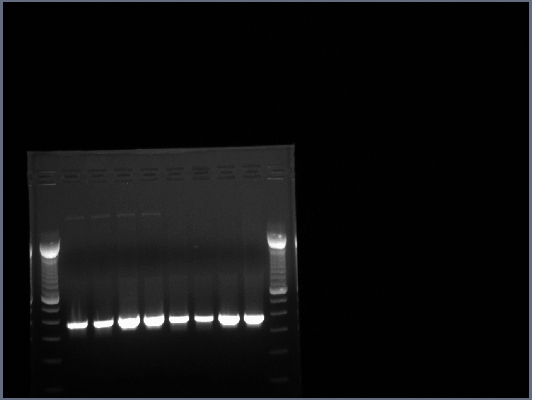
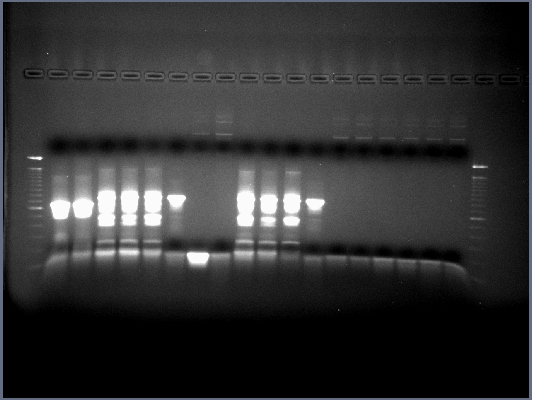
1.5% agarose gel made, 0.75g agarose in 50ml 0.5X TBE w/10ul EtBr.
20ul of PCR produts loaded on gel w/4ul 6X loading buffer, run at 200V for about half an hour.
All lanes had lots of DNA at ~375bp which is the expected length, PCR worked.
July 31, 2008
Another PCR to asemble the TE33 heavy and light chain fragments from yesterday. The complementary region codes for a flexible glycine-serine linker that will join the two halves of the single chain antibody.
| tube | 1 | 2 | 3 | 4 | ||||
| template from 7-30 PCR | 2+3 | 2+7 | 6+7 | 1+8 | ||||
| template | 0.5ul of both heavy and light chains | |||||||
| primers | 1ul of appropriate forward and reverse primer | |||||||
| MgCl2 | 0ul | 0ul | 3ul | 3ul | ||||
| master mix | 20ul | |||||||
| H20 | to 50ul total volume | |||||||
PCR program
- 94 degrees 5 min
- 94 degrees 1 min
- 50 degrees 1 min
- 72 degrees 1 min
- GOTO 2. 29 cycles
- 72 degrees 5 min
- HOLD at 4 degrees
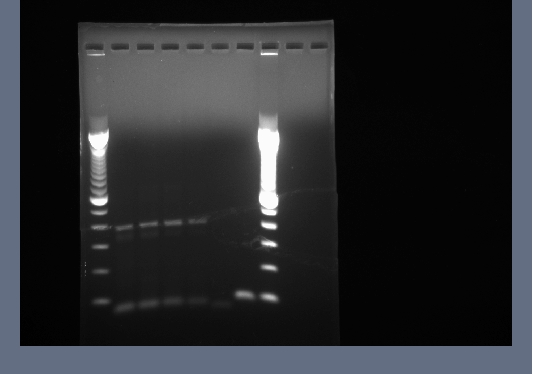
Products run on a 1.5% agarose gel, no products in the expected 700-800bp range.
August 1, 2008
PCR troubleshooting of yesterday's reaction: use gradient for annealing temp (40-65 degrees), use more template, leave out end primers
Four series of reactions using a gradient annealing temp were run in addition to a fifth series omitting the end primers.
| reaction series | A | B | C | D | E |
| template (5+7) from 7-30 PCR | 0.5ul | 1ul | 1.5ul | 2ul | 1ul |
| primers | 1ul of forward and reverse primers (omitted in E series) | ||||
| master mix | 20ul | ||||
| H20 | to 50ul total volume | ||||
Annealing temps: 40, 43.8, 50, 54, 60, and 64 degrees
PCR program
- 94 degrees 5 min
- 94 degrees 1 min
- annealing step 1 min
- 72 degrees 1 min
- GOTO 2. 29 cycles
- HOLD at 4 degrees
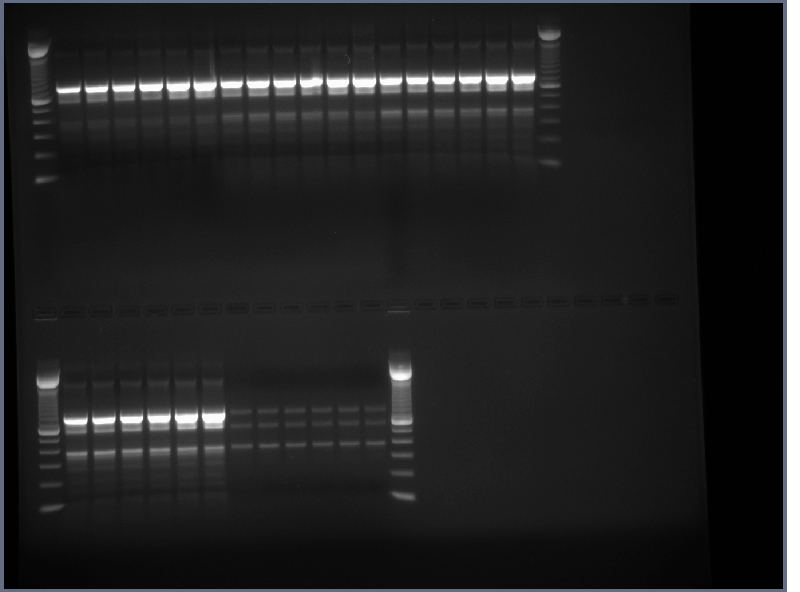
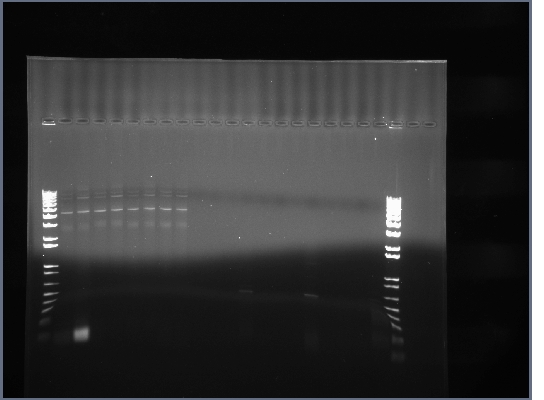
PCR products run on 1.5% agarose gel at 200V for ~20 minutes, then 240V for ~20 minutes.
Products in the 700-800bp range in all lanes. Perhaps it worked because of using a differnt product from the 7-30 PCR. We now have the DNA for our single chain antibody.
August 6, 2008
1.5% agarose gel of 8-1 PCR products run to cut out bands. Products A1, B1, C1, and E1 used. We will use this purified DNA for making biobricks and future PCRs to assemble our antibody-receptor fusion.
Bands around 800bp cut out for all lanes and invitrogen gel extraction kit used to collect DNA in 50ul H2O.
September 5, 2008
Flk-1 clones from [http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx?ATCCNum=MGC-18600&Template=mgcMouseClones ATCC] streaked onto LB+amp and incubated at 37 degrees.
Flk-1 is a mouse gene coding for a vascular endothelial growth factor receptor, a tyrosine kinase receptor. We chose this particular receptor because it is well characterized and because it appears to activate simply through dimerization. This is important because if ligand binding caused a conformational change that helped to activate the receptor it is unlikely that dimerization caused by the single-chain antibody binding cholera toxin would create a similar conformational change.
September 6, 2008
Colonies picked from flk-1 plate and inoculated into 5ml LB+amp, grown at 37 degrees.
Freeze-dried e. coli with YCp50-poly from [http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx?ATCCNum=87555&Template=vectors ATCC] resuspended in 5ml LB, grown at 37 degrees.
YCp50-poly is the yeast/E. coli shuttle vector we chose to place our receptor gene on. It allows for selection using ampicilin in E. coli and media lacking uracil in yeast.
September 7, 2008
Mini spin prep of flk-1 and YCp50-poly cells, plasmids stored in 50ul H2O.
We now have purified supplies of the plasmid carrying the flk-1 gene and our shuttle vector.
September 25, 2008
Biobricks
Here we ran PCRs to include the biobrick prefix and suffix sequences into the genes for the TE33 heavy and light chains, the single-chain antibody, and flk-1. PCRs were also run to amplify various lengths of the flk-1 gene. We want to investigate whether certain sections of the extracellular domain of flk-1 are important for activity, or if much of the extracellular domain can be replaced by the single-chain antibody. Flk-1 links 1, 2, and 3 delete 222,
| tube | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 0.5uL template | H chain | L chain | SCA | SCA | SCA | SCA | flk-1 | flk-1 |
| IDT | IDT | A | B | C | E | A | B | |
| primers | 1uL forward & reverse for all | |||||||
| mastermix | 8.25uL for all | |||||||
| H2O | 39.25uL for all | |||||||
| tube | 9 | 10 | 11 | 12 | 13 | 14 | ||
| 0.5uL template | SCA w/ link | SCA w/ link | SCA w/ link | SCA w/ link | flk link 1 | flk link 1 | ||
| A | B | C | E | A | B | |||
| primers | 1uL forward & reverse for all | |||||||
| mastermix | 8.25uL for all | |||||||
| H2O | 39.25uL for all | |||||||
| tube | 15 | 16 | 17 | 18 | ||||
| 0.5uL template | flk link 2 | flk link 2 | flk link 3 | flk link 3 | ||||
| A | B | A | B | |||||
| primers | 1uL forward & reverse for all | |||||||
| mastermix | 8.25uL for all | |||||||
| H2O | 39.25uL for all | |||||||
PCR program
- 94 degrees 4 min
- 94 degrees 1 min
- 50 degrees 1 min
- 72 degrees 3 min
- GOTO 2. 29 cycles
- HOLD at 4 degrees
September 26, 2008
- product from yesterday run on 1% gel, 200V for ~30 minutes
- mostly good results, small amound of DNA for flk-1
- lanes 1,2,6,8,12,14,16,18 cut out and purified with invitrogen gel extraction kit, eluted in 50ul H2O
PCR to assemble SCA-flk-1 fusion and increase flk-1 yield
| tube | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| template | 0.5ul flk-1 A | 0.5ul flk-1 B | 0.5ul flk link 1 A | 0.5ul flk link 1 B | 0.5ul flk link 2 A | 0.5ul flk link 2 B | 0.5ul flk link 3 A | 0.5ul flk link 3 B | 0.5ul SCA link + 0.5ul flk link 1 | 0.5ul SCA link + 0.5ul flk link 1 (crude) | 0.5ul SCA link + 2ul flk link 1 | 0.5ul SCA link + 2ul flk link 1 (crude) | 0.5ul SCA link + 0.5ul flk link 2 | 0.5ul SCA link + 0.5ul flk link 2 (crude) | 0.5ul SCA link + 2ul flk link 2 | 0.5ul SCA link + 2ul flk link 2 (crude | 0.5ul SCA link + 0.5ul flk link 3 | 0.5ul SCA link + 0.5ul flk link 3 (crude) | 0.5ul SCA link + 2ul flk link 3 | 0.5ul SCA link + 2ul flk link 3 (crude) |
| primers | 1uL forward & reverse for all | |||||||||||||||||||
| mastermix | 8.25uL for all | |||||||||||||||||||
| H2O | to 50ul total volume | |||||||||||||||||||
PCR program
- 94 degrees 4 min
- 94 degrees 1 min
- 50 degrees 1 min
- 72 degrees 3 min
- GOTO 2. 29 cycles
- HOLD at 4 degrees
September 27, 2008
- 0.8% agarose gel run of products from yesterday, 200V for ~45 minutes
- first 8 reactions may have worked but bands not great, assembly reactions did not work
September 28, 2008
0.8% gel run of yesterday's products, 150 V, 38 min, 200 V, 10 min
September 30, 2008
p1010 plasmid for biobicks cut out of plate 1003, well 4A; soacked in 5uL warm TE (50°C) for ~20 min, heat shocked at 50°C for 60s, placed on ice 2 min, 200uL LB added, incubated at 37°C, 1 hour, plated on LB + Amp and incubated overnight.
Restriction digest of our biobricks 18uL H2O 2uL 10x buffer Z 1uL EcoRI and PstI 1,2,4 uL DNA (H chain, L chain, SCA, flk)
p1010 transformatoin was stupid, transformed another plasmid instead, 1008, 1C
| tube | 1 | 2 | 3 | 4 | 5 | 6 | ||
| template | A=0.5uL B=1.0uL C=1.5uL | (all flk-1 B) | ||||||
| primers | 1uL forward & reverse for all | |||||||
| mastermix | 8.25uL for all | |||||||
| H2O | 39.25uL for A | 38.75uL for B | 38.25uL for C | |||||
| tube | 1 | 2 | 3 | 4 | 5 | 6 | ||
| gradient | 55.0 | 57.8 | 59.3 | 61.0 | 63.5 | 65.0 | ||
| temps | (1) | (5) | (6) | (7) | (9) | (12) | ||
Run for 39 cycles
October 2, 2008
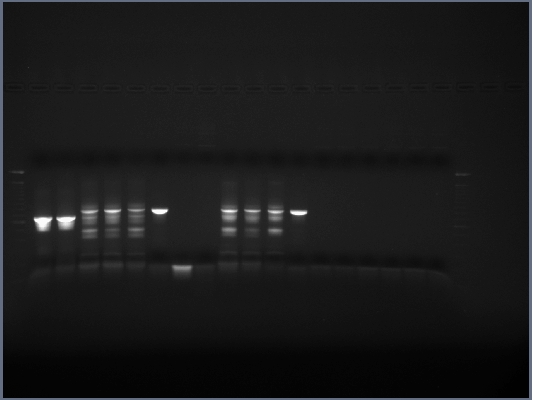
PCR products run on 0.8% gel, 200V, ~40min Same results as always
pSB1A3 & pSB1A7 cut out of registry and transformed Plated on LB+Amp
October 3, 2008
PCR from yesterday may have kind of worked, just not a lot of product, running another gel to find out
Transformation failed, transforming w/ RDR plasmid to test transformation protocol
Hard to tell if PCR products are correct length, so-so bands
October 8, 2008
Transformations to get plasmids 1003:2F, 1008:10D, 1008:1C, 1008:6G
October 10, 2008
DNA purification using S prime PerfectPrep(TM) Spin Mini kit on transformed bacteria from 10/8/2008
October 14, 2008
Transformation using plasmid from 10/10/2008
October 16, 2008
Transformation failed, duh top 10 50uL cells transformed with 1003:10D, 1003:2F, plasmid from 10/10/2008, and pUC19 control
PCR to make ARTK fusion
| tube | 1 | 2 | 3 | 4 | 5 |
| template | 0.5uL SCA + 0.5uL flk-link3(8) | 0.5uL SCA + 1.0uL flk-link3(8) | 0.5uL SCA + 1.5uL flk-link3(8) | 0.5uL SCA + 2.0uL flk-link3(8) | 0.5uL SCA + 1.5uL flk-link3(8) |
| primers | 1.0uL forward & reverse | none | |||
| mastermix | 20.0uL for all | ||||
| H2O | 27.0uL | 26.5uL | 26.0uL | 25.5uL | 28.0uL |
October 17, 2008
Transformation failed. 1% gel of yesterday's PCR run, 200V, ~40min, no bands
October 18, 2008
Using phusion to get ARTK
| tube | 1 | 2 | 3 | 4 | 5 |
| template | 0.5uL SCA + 0.5uL flk-link3(8) | 0.5uL SCA + 1.0uL flk-link3(8) | 0.5uL SCA + 1.5uL flk-link3(8) | 0.5uL SCA + 2.0uL flk-link3(8) | 0.5uL SCA + 1.5uL flk-link3(8) |
| primers | 1.0uL forward & reverse | no primers | |||
| buffer | 10.0uL for all | ||||
| phu | 0.5uL for all | ||||
| H2O | 36.5uL | 36.0uL | 35.5uL | 35.0uL | 37.5uL |
October 21, 2008
Made 500ml LB media 500mL DI water 5g Tryptone 2.5g Yeast Extract 2.5g NaCl Autoclaved on liquid setting, 15 min.
Frozen stocks of pSB1A7 and pSB1A3 from iGEM used to innoculate LB+Amp cultures for plasmid prep.
October 22, 2008
Cultures from yesterday used for plasmid prep (minispin kit)
restriction digests
| tube | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 5uL DNA | pSB1A7 | pSB1A3 | H brick | L brick | SCA brick | flk-1 brick | flk-1 plasmid |
| 5uL buffer2 | |||||||
| 0.5uL BSA | |||||||
| 1uL RE 1 | EcoRI | ||||||
| 1uL RE 2 | PstI | HindIII | |||||
| 37.5uL H20 | PstI | HindIII | |||||
| 37.5uL H20 | |||||||
October 23, 2008
Transformations failed; competent cells may be bad. Innoculated 5mL culture with DHS & PRO to make more competent cells tomorrow.
Ran 0.8% gel of restriction digests, no band in H brick lane (!) or flk-1 brick (expected), the rest as expected. Cut out * purified bands for ligations and PCR.
PCR to get flk-1 fragments 0.5mL purified flk-1 fragment, 1uL F & R primers, 20uL matermiz, 27.5uL H20
| tube | 1 | 2 | 3 | 4 |
| flk-1 | flk-1 link 1 | flk-1 link 2 | flk-1 link 3 |
| Home | Team | Project | Notebook | Research Articles | Parts | Protocols | Pictures |
 "
"