Team:Hawaii/Initial Synth. Oligo Assembly
From 2008.igem.org
Contents |
Overview
Construct the nir promoter and slr2016 and pilA signal sequences in Biobrick format and clone them in E. coli as part 1 of cyanobacterial secretion system development.
Order of events:
- Synthesize oligonucleotides encoding the nir promoter and slr2016 and pilA signal sequences in Biobrick format.
- Anneal sense and anti-sense oligonucleotides.
- Ligate oligonucleotides to create the full Biobrick parts.
- Restriction digest the ligation products.
- Run annealed and digested ligated products on a gel to verify annealing and ligation.
- Extract bands corresponding to the correct ligated products from the gel.
- Ligate the nir promoter and slr2016 and pilA signal sequences to a vector.
- Subclone in E. coli.
Protocol
Hybridization of Parts
- Mix:
- 3 μl 100 µM sense oligo
- 3 μl 100 µM anti-sense oligo
- 3 μl 10 x PNK (polynucleotide kinase) buffer
- 2 μl 10mM ATP
- 2 μl T4 polynucleotide kinase (PNK)
- 17 μl distilled water
- Total volume = 30 μl
- Incubate at 37C for 1.5 hours.
- Add 4 μl 0.5 M NaCl.
- Place in boiling water bath for 2 min., then remove water bath from the heat source and allow the reaction (still in the water bath) to cool to room temperature (approx. 30 minutes)
- Reference: Pam Silver Lab. [http://openwetware.org/wiki/Silver:_Oligonucleotide_Inserts| Oligonucleotide Inserts.]
DNA Ligation
- Combine 4 ul of construct one with 4 ul of construct 2.
- Add 1 ul of nanopure H20.
- Add 10 ul of 2x quick ligation reaction Buffer. In order to avoid shearing the DNA, mix by resuspending very slowly.
- Add 1 ul of Quick T4 DNA ligase and mix thoroughly by resuspending very slowly.
- Incubate at room temperature for 5 minutes.
- Cool on ice then transform, or store at -20oC
- Reference: Quick Ligation Kit from NEB.
Restriction Enzyme Digest of Ligated Oligonucleotides
- May not be necessary
- Added 7 μl nanopure water, 1 μl NEBuffer 3, 1 μl XbaI, and 1 μl PstI to the 20μl ligation reaction.
- Incubated 1 hour at 37C.
- Stopped reaction by incubating 10 min. at 65C.
Note:
- This reaction did not follow the reagent amounts recommended by NEB or "Short Protocols" because the ligation buffer contained ingredients not usually found in DNA solutions to be digested. Dr. Presting revised the recommended protocols to adjust for this factor.
- Reference: Short Protocols in Molecular Biology. Vol. 1. 5th edition. 3-3 to 3-4.
Restriction Enzyme Digest of BBa_C0012
- Combined 0.4 μl C0012 (from plasmid prep), 15.6 μl nanopure water, 2 μl 10X NEBuffer 2, 1 μl XbaI, and μl PstI.
- Incubated 2.5 hours at 37C (recommended incubation of at least 1 hour)
- Stopped reaction by incubation 10 min. at 65C.
General Notes on RE Digests:
- Remember to check that REs are compatible.
- Reaction can also be stopped by adding 5μl 10X loading buffer.
- <10% of digest should be RE
- REs are stored in glycerol. <5% glycerol should be present in digest. Therefore, a minimum 10-fold dilution is necessary so the enzymes don't act funky.
- It's always a good idea to gently shake and spin down RE and RE buffer prior to use.
- References: Short Protocols in Molecular Biology. Vol. 1. 5th edition. 3-3 to 3-4.
- NEB
- References: Short Protocols in Molecular Biology. Vol. 1. 5th edition. 3-3 to 3-4.
Gel extraction
- Used Qiagen MiniElute Gel Extraction kit.
Transformation into DH5α
- Incubated 8 minutes on ice instead of 30 minutes. Dr. Callahan recommended 5-10 min. incubation because longer incubations may decrease the number of transformants.
Results
First attempt
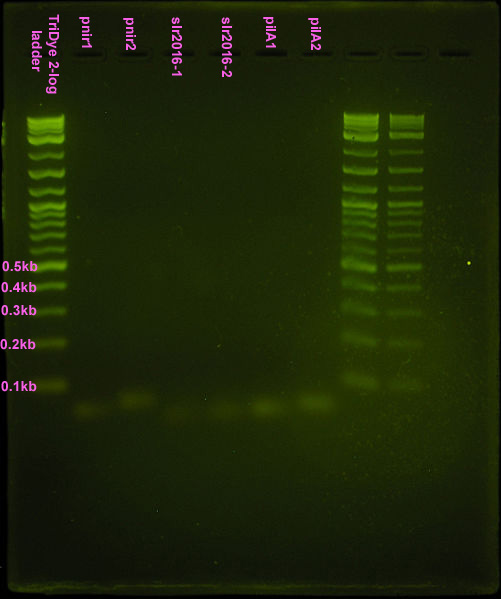
Nice bands observed for annealed oligos. Bands appear to be the correct size (<100bp) but we can't tell if anneal worked (EtBr doesn't tell us if these are ssDNA or dsDNA bands). Smear is probably from loading way too much DNA (see discussion). Possible faint band for pnir ligation. No bands for slr2016 and pilA ligation, probably because way too much DNA was added for the ligation reaction.
Second attempt
Did a ligation using 1 μl of a 10-6 dilution of each annealed product. Ligated at both room temperature and at 40C (recommended by Frank, etal). Nothing was visible on the EtBr stained 4% agarose gel because not enough DNA was present.
Third attempt
Did a ligation using 10-2 dilutions of annealed product (ligated at room temperature and at 40C). Faint bands were visible for the annealed products (10-2 dilutions) but no bands were observed for the ligated products. Not enough DNA was present. Ran 10-1 dilutions of annealed product on SYBR Safe stained 2% agarose gel.
Fourth attempt
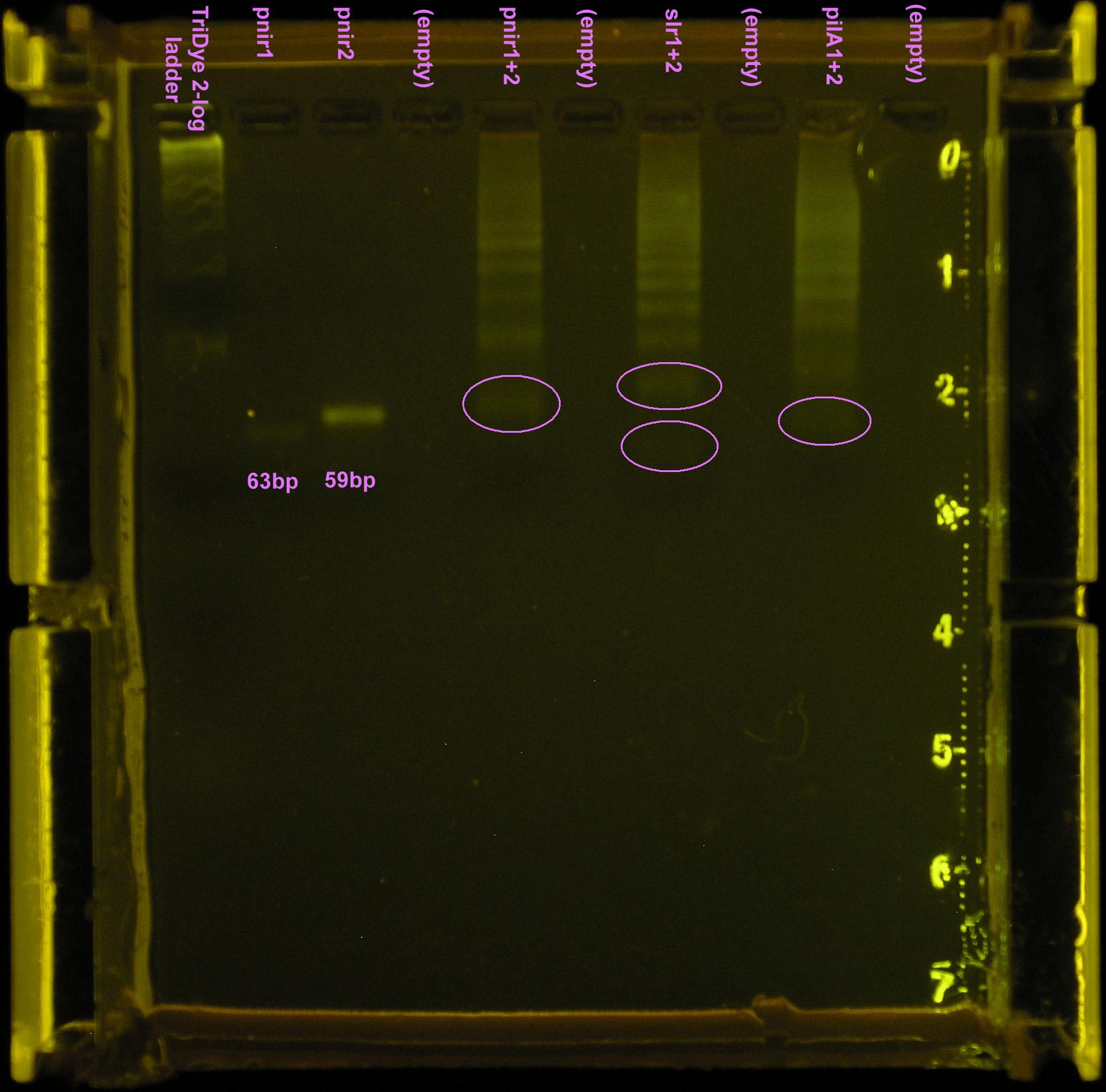
Took 10 μl ligation reaction from first attempt and restriction digested with XbaI and PstI (1 μl each) in NEBuffer 3 (1 μl). A ladder of bands were observed for the ligation product, indicating incomplete digestion.
Fifth attempt
Repeated ligation using full concentrations of annealed product. RE digested as in fourth attempt, with incubation for 2.5 hours. Loaded 20 μl of ligation into well with 2μl loading dye. Less loading dye was used so the bromophenol blue would not block out our band. Band(s) will not resolve on the gel if less than 20μl of the ligation is loaded.
Gel turned out funky (was it poured too late and the agarose already began solidifying?) Ladder did not run correctly but luckily, the pnirs did (we know their size). Also, from the fourth attempt gel, we knew the approximate location of ligation bands. Bands excised from the gel are circled in the figure below. For the slr2016 signal sequence ligation, no band was observed at the expected size. Very faint bands were observed above and below the expected ~80bp mark so both were excised and purified.
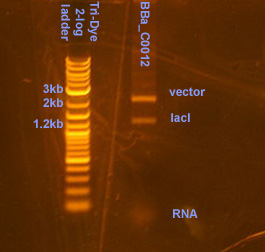
Plasmid prep and digestion of BBa_C0012 harboring vector
The plasmid prep was RE digested using XbaI and PstI per NBE's instructions. Distinct bands were observed the gel at 1.2kb and 2.1kb, corresponding to the lacI gene (BBa_C0012) and the vector. Comparing the intensity of the vector band to the ladder bands, it is estimated that there is ~40ng of vector.
Transformation into DH5α
No transformants were observed.
Sixth attempt
The ligation was repeated because the gel from the 5th attempt was of questionable quality and no transformants were observed. Three different constructs of the full nir promoter and slr2016 and pilA signal sequences were created. The first followed the ligation and restriction digest protocols previously used, using full concentrations of annealed products. The second was a ligation and restriction digest carried out using 10-1 dilutions of annealed product. It was suggested by SC that if too much DNA was added to the ligation mixture, "DNA hairballs" would form between ssDNA (unannealed) and dsDNA, resulting in smears and multiple bands in the gel. The last method of construction simply placed equal concentrations of annealed products together. In theory, DNA overhangs would anneal appropriately, yielding an unligated but full promoter or signal sequence.
Ligation at full concentrations of annealed product worked best. The strongest bands were observed for this method. Multiple bands were still observed, above and below the expected band length (80-100bp) indicating both unligated DNA fragments and incompletely digested DNA or hairball structures. However, the strongest band for all three constructs corresponded to the desired product. The 10-1 ligations were barely visible on the gel, and multiple bands were still observed, so ligation and restriction digest efficiency was not improved by the addition of less DNA. The last method of annealing but not ligating yielded two bands in the gel corresponding to the two original DNA fragments.
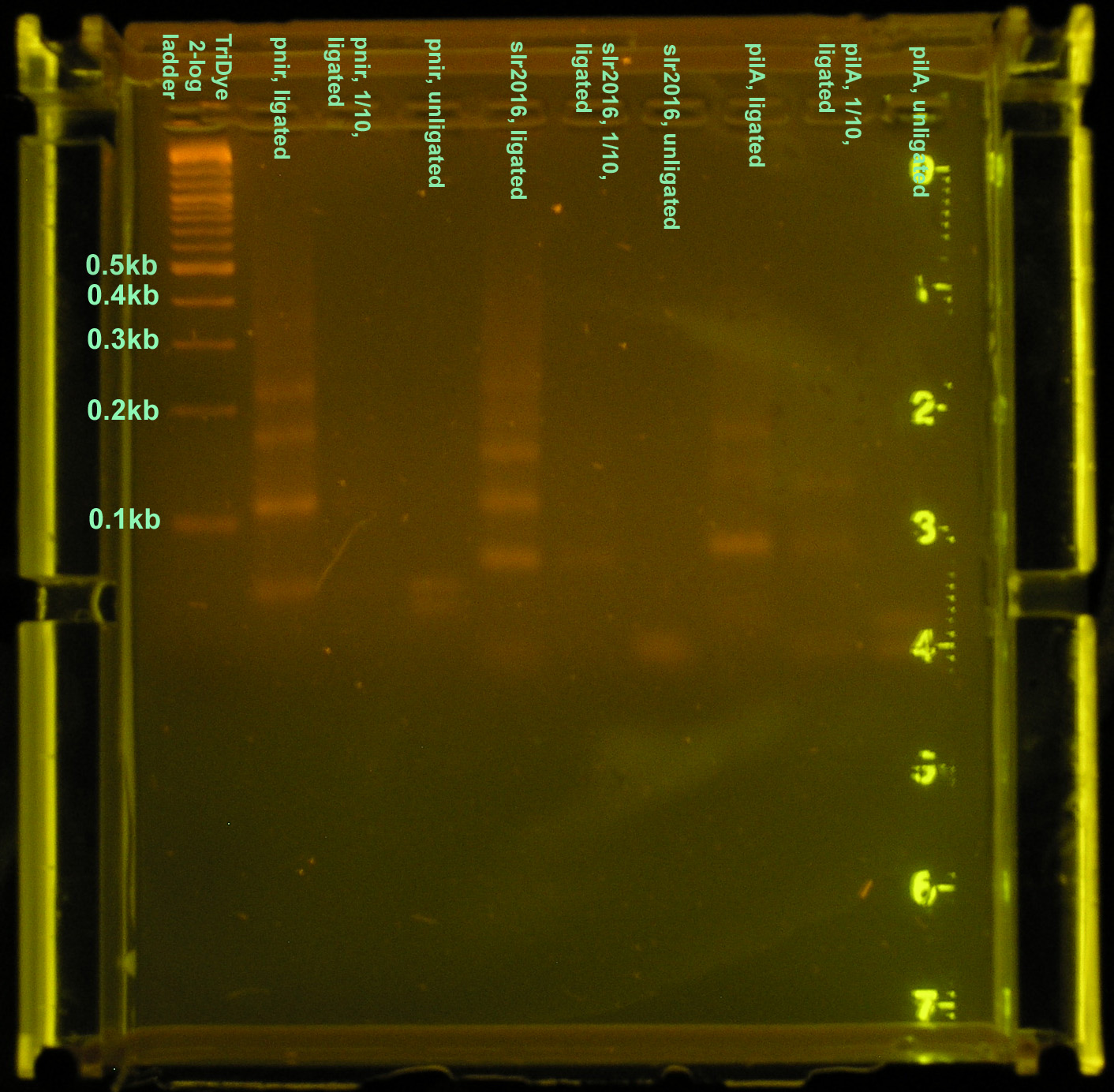
A brand new, pure, agarose gel was run again using the products of the same reactions. Twenty microliters of product were loaded into each well and bands corresponding to the desired products were excised and purified. This gel ran at 60V for 2 hours for better band separation. Bands were twice as bright as before and farther apart, but otherwise identical to the gel pictured.
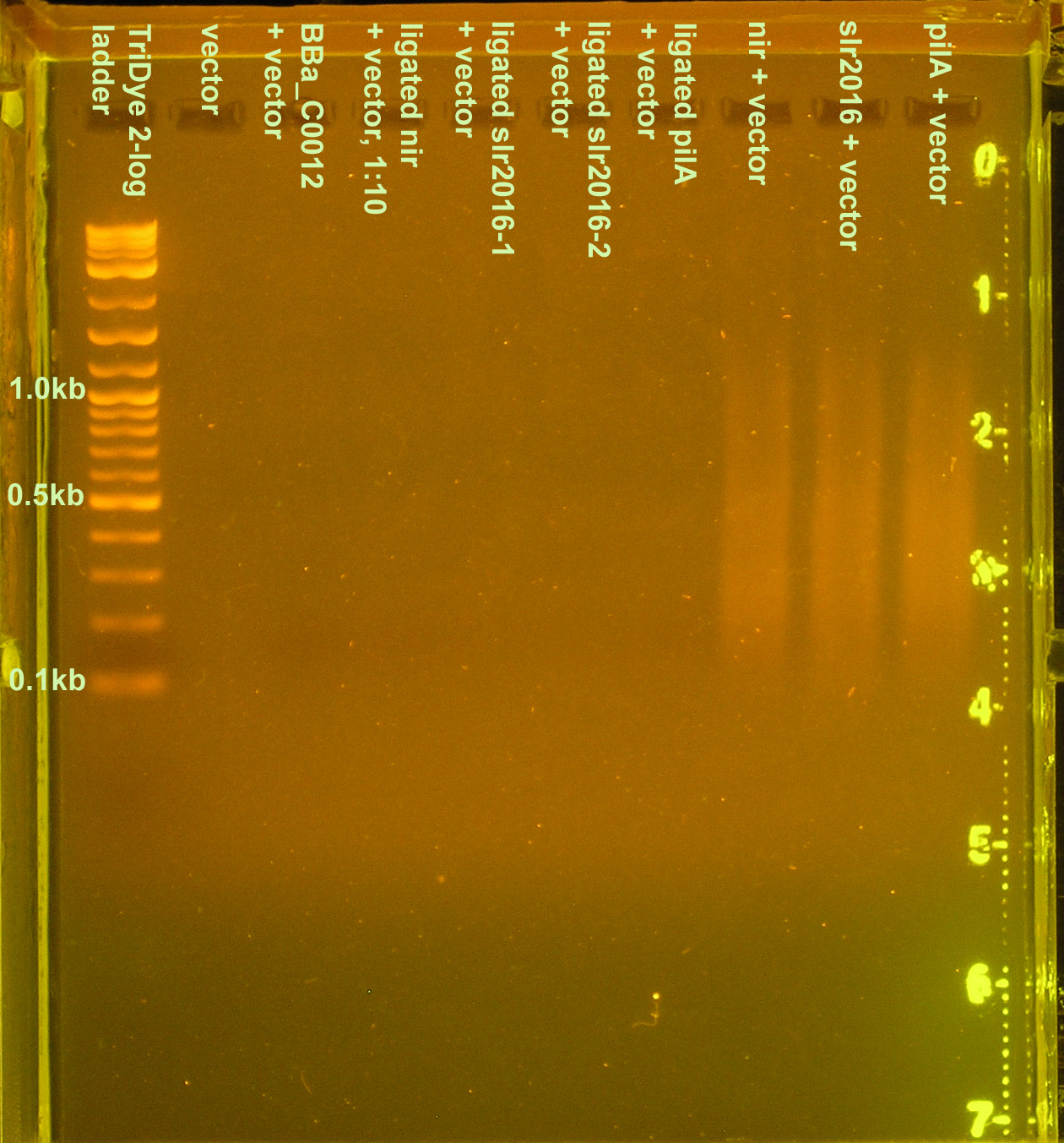 The purified DNA was then ligated to a Biobrick vector for subcloning in E. coli. Ligations were carried out using 3:1 molar concentrations of insert to vector. Ligations were performed using full concentrations of insert and vector as well as 1:10 dilutions of both. A third method used full concentrations of annealed product and vector. When ran on a 3% agarose gel for verification of ligation, only products from the third method were visible. Again, a smear similar to that seen in the first ligation attempt (see above) was observed indicating "DNA hairballs." The 1:1 dilutions ran were probably not visible because the DNA concentration was too low, despite the fact that 15 μl (out of 20 μl) of the ligation mixture was loaded.
The purified DNA was then ligated to a Biobrick vector for subcloning in E. coli. Ligations were carried out using 3:1 molar concentrations of insert to vector. Ligations were performed using full concentrations of insert and vector as well as 1:10 dilutions of both. A third method used full concentrations of annealed product and vector. When ran on a 3% agarose gel for verification of ligation, only products from the third method were visible. Again, a smear similar to that seen in the first ligation attempt (see above) was observed indicating "DNA hairballs." The 1:1 dilutions ran were probably not visible because the DNA concentration was too low, despite the fact that 15 μl (out of 20 μl) of the ligation mixture was loaded.
Transformation into DH5α...
Discussion
We need to dilute our annealed products before running them in a gel or attempting ligation. 20-125ng DNA (per lane) is good for running gels. One reaction using the NEB Quick Ligation Kit can ligate up to 180ng of DNA.
After the gel extraction, we end up with very little product to transform with. In the future, we should run larger ligation reactions of annealed product and perhaps run 2-3 lanes of it in the gel.
 "
"