Team:KULeuven/24 July 2008
From 2008.igem.org
| << return to notebook | return to homepage >> | ||
| < previous friday | ← yesterday | tomorrow → | next monday > |
Contents |
Lab Work
Wet Lab
- Stefanie and Hanne made a glycerolstock of all the parts we received from the iGEM headquarters. Everything is now stored in two -80°C freezers. You can find the cells with the parts in box S&PF7. You can look up in the cmpg database where you should search for a specific part.
In the afternoon we started the protocol to make the right E.coli strain. We made a liquid culture of the donor and acceptor cells. Phage P1 was incubated with the acceptor. Tomorrow we'll continue the proces and bring phage P1 and the acceptor cells (MC0106) together, this should result in the right cells.
- Because the concentrations of the isolated plasmids were low yesterday, we now put a bigger volume of cells over the mini-prep column. We did it for the following BioBricks:
- B0015
- B0032
- B0034
- E0022
- I714891
- J23100
- M30109
- R0084
Then the plasmids were digested with the restriction enzymes (EcoR1, Spe1, Xba1) and put on an agarose gel. The plasmids with BioBricks I714891 and Xxxxx were not cut.
Dry Lab
Primers for the construction of T7 polymerase with UmuD tag were written out.
Modeling
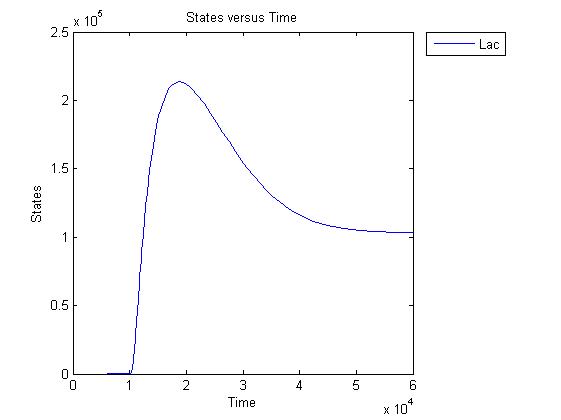
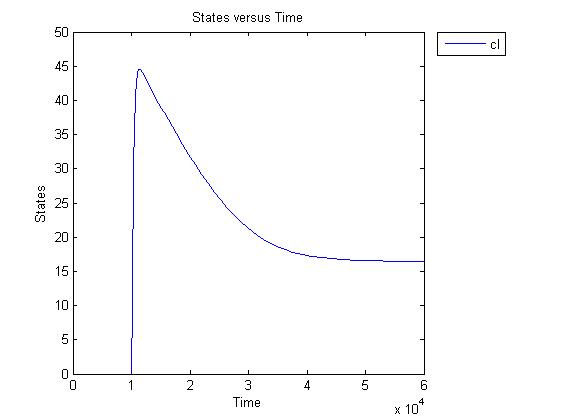
Pulse generator has been reviewed, the parameters have been checked but there are issues... the pulse generator doesn't work (pure physically): when a step signal of T7 is applied cI is produced, which will then repress its transcription. Of course, this will develop to an steady state with a cI level equal to a non zero value.
A zero cI value would result in zero repression, thereby restarting the transcription of the cI gene and start producing cI again anyway.
Because the cI value remains non zero, so will the lactonase production, and so there will always be a constant amount of lactonase present. Question is how much this concentration will affect the system considering the case of no light present. No light present means HSL production which should bind to LuxR, but instead will react with lactonase to an hydroxy-acid. If the concentration of lactonase is considerable, and remains somewhat steady, cell death would never occur.
| 
|
Interesting link to a pulse generator system that seems to work: Pulse Generator Keasling Lab
The memory system has been reviewed as well. The old system seemed unsuccesful, a new system has been found proven to work. There are however not enough repressors available to implement this system without having interference with other parts. ...
Wiki has been updated a bit more. SBMLSqueezer was used to produce pdf files with the celldesigner reactions, species and parameters. Biobricks pictures were added and some other stuff.
Remarks
iGEM gave us plasmids in cells. Which cells?
Quote of the day
GAS!
 "
"