Team:Cambridge/Signaling/Experiments
From 2008.igem.org
Contents |
Finding and Biobricking proper Ribosomal Binding Sites
- [http://partsregistry.org/Assembly:RBS-CDS_issues Solving Biobrick RBS issues]
Testing Sender and Reciever
- We can test one, then use to test the other, or we can test them in parallel
Testing Agr Sender
A number of options have been considered to measure the AIP output of our sender construct.
- Using Ellman's reagent to detect thiol groups
- Mass Spec
- Using a biological reporter
The last option is determined to be most feasible. A novel lux-based agrP3-reporter fusion S aureus strain (R0J48) was kindly provided by Rasmus Jensen of Nottingham University (here's the link to the paper http://www.ncbi.nlm.nih.gov/pubmed/18582472). In this strain, the agrABCD locus is knocked out using TetM and lux and the luxABCDE operon inserted downstream of the p3 promoter. agrA and C1 are subsequently reintroduced under the p2 promoter in a plasmid pAgrC1A. Growth conditions:
- S aureus can be grown in LB or agar plates at 37 degress. The paper suggested the following selection: 10μg/mL Cm, 5μg/mL Ery, 5μg/mL Tet. However, TetM is under control of P2 so expression in the absence of AIP may be too low for TetR. Also, it is unclear how CmR is acquired.
- Some papers seem to prefer CYGP broth (10 g/liter casamino acids/10 g/liter yeast extract/5 g/liter glucose/5.9 g/liter NaCl/60 mM β-glycerophosphate). (Novick R P. Methods Enzymol. 1991;204:587–636. [PubMed])
- Dilute overnight cultures 1/20 in fresh medium and incubate for 2 h.
- Dilute 1/50 into 96 well plate with dilution series of AIP.
- relative light unit per cell density (RLU/OD492) taken every 30 min for 24 h. Peak value used for each dilution series.
- This can be compared to standard curves generated from known concentraions of AIP given in the paper (fig3). The EC50 of the reporter for AIP-1 is 6±1.
Until the sender construct is made, we plan to test the reporter strain using alternative sources of AIP. A number of options have been considered:
- Chemical synthesis (too difficult)
- Purification using a mini-intein fusion (http://aem.asm.org/cgi/content/abstract/73/19/6036)
- Purification from S.aureus supernatant.
Again, the last option is most suitable for our investigation. Here's the protocol taken from Balaban et al (http://www.jbc.org/cgi/content/abstract/276/4/2658)
- Post exponential culture (around 200ml) centrifuged at 6000g for 10 minutes at 4°C.
- Supernatant collected and filtered through 0.22μm to remove residual cells.
- Filtrate lyophilized and resuspended in water to one-tenth original volume (10 fold concentrated)
- 15ml supernantant applied to 3-kDa cutoff membrane.
- Filtrate should contain AIP (can be stored at -80°C). Test by applying 50μl of each 10 fold dilution series to 450μl reporter.
Testing AgrC Reciever
- Use purified AIP
- Synthesized
- Sourced
- Possibly from Tom Muir at Rochester
- S. aureus purified supernatant (requires Pathology department personnel)
- Guangyong Ji at Catholic University has a protocol for purification
Testing AgrA Transcription Factor and P2 promoter
- Can we get an 'always on' mutant my modifying some residues? (Jim's Idea)
Testing AgrB Exporter
- Membrane Localization Test
- Can use membrane fractionation
- Can use antibody tags with EM and gold
Testing AgrD Prepeptide/AIP
- Mass Spec Assay
- Daniel Goodman 11:47, 31 July 2008 (UTC):Speaking to Kathryn Lilley in Proteomics
- S. aureus Reporter Strain
- Daniel Goodman 11:47, 31 July 2008 (UTC):We cannot use staph ourselves, checking to see if we can get someone in Pathology department to test for us
Testing AHL sender and receiver
Testing AHL receiver (T9002)
- Spot 10 μl 10μM AHL on receiver-soft agar overlay and test for fluorescece. Result: postive response.
- Spot a range of AHL concentrations (10μM, 1μM, 10nM, 1nM)for a qualitative dose-response test. Result: can detect down to 1μM AHL using UV lamp, but response is poor.
- The above test is re-run this time using fresh, log-phase receiver cells (2 hours incubation after inoculation) and a different set of AHL concentrations (10μM, 1μM, 100nM, 10nM). Detection much higher for 10μM and 1μM. Observable response to 10nM although signal-to-noise ratio is poor.
Testing AHL sender (I13202)
- Sender grown overnight from plate in 2*10 ml LB. Receiver grown overnight from plate in 10 ml LB.
- 3 Amp plates made with receiver-SA overlay (see above). In addition, the SA overlay of the 4th plate contains 1mM IPTG.
- 10 ml of sender culture used for plates 1,2 and 4.
- Plate 1: spot 10μL sender on the center of the plate.
- Plate 2: Spot 10μL sender premixed with IPTG (final concentration of 1mM). Spot immediately after mixing.
- Plate 4: Spot 10μL sender (plate contains IPTG)
- 10 ml of sender culture pelleted and resuspended in LB containing 1mM IPTG. Grow for 2 hours.
- Plate 3: Culture pelleted again and 10μL supernatant, which contains AHL, spotted onto plate.
- Result
- All plates, including plate 1, had high levels of fluorescence, detectable by a UV lamp. This confirms that the lacI+pL hybrid promoter (R0011) is constitutively on at a high level (http://partsregistry.org/wiki/index.php?title=Part:BBa_R0011). However, there is no evidence for higher fluorescence in the presence of IPTG despite claims that a 600 fold increase in expression should occur. This may be because the concentration of sender is too high.
- The properties of lacI+pL, though interesting, is not of concern for our project, as we intend to use another Bacillus compatible promoter anyways.
- This experiment has confirmed that the sender is definitely capable of secreting AHL, which is sufficient for this stage.
- Future work:
- A lower concentration of sender could be used either by dilution or lower growth times.
- for better quantification, we could try a protocol similar to the one used by the NCBS 2007 team.
Testing degradation of AHL by bacillus aiiA
- 5 plates without selection with E.coli receiver overlay in soft agar. Center of plate spotted with:
- B subtilis in LB and AHL in EA medium without incubation
- B subtilis in EA medium only (control for competition between B.subtilis and E.coli)
- AHL only
- B.subtilis and AHL incubated for 90 minutes at room temperature
- B.subtilis in overnight growth medium (test for effect of acidity on AHL) and AHL in EA.
- Plates grown overnight
- Result: inconclusive. All plates except plate 2 (no AHL) had similar levels of fluorescence. No fluorescence in plate 2. This may because our Bacillus strain (168) is a aiiA knockout, as no aiiA gene was found in the genome. However, at 5 hours after incubation, only plate 3 (no Bacillus) had detectable levels of fluoresence.
Testing different volumes of AHL
Our previous experiments were inconclusive because we believed that we possibly swamped our Bacillus with excess amounts of AHL so that no appreciable degradation was seen. In this experiment we tested and compared different concentrations of AHL.
Fluorescence with 1 uM AHL
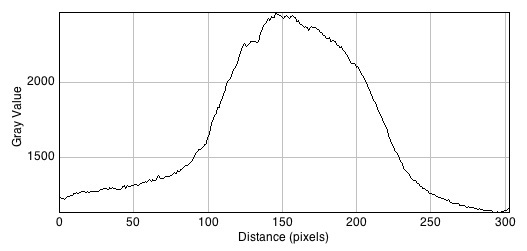
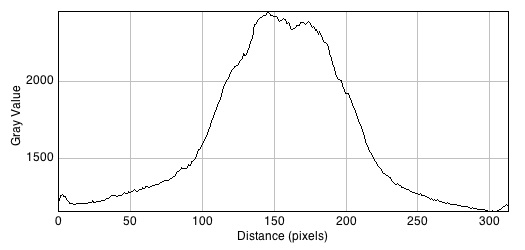
- Without Bacillus: 2457
- With Bacillus: 2497
Fluorescence with 10nM AHL
- Without Bacillus: 1424
- With Bacillus: 1318
Fluorescence with 1nM AHL
- not enough for good readings
Images of 1uM AHL plates with accompanying plot profiles
The graphs demonstrate fluorescence change across the plate from left to right.
 "
"