Team:Utah State/Project
From 2008.igem.org

|
| Home | The Team | The Project | Parts | Notebook | Protocols | Links |
|---|
Contents[hide] |
Abstract
The Utah State University iGEM team project is focused on creating an efficient system for production and monitoring PHA production in microorganisms. One goal of our research is to develop and optimize a method, using fluorescent proteins, for the detection of maximum product yield of polyhydroxybutyrate (PHB, a bioplastic) in recombinant E. coli and in Cupriavidus necator. In order to develop an optimal PHB detection system, we focused on the identification of the most efficient reporter genes, and the best promoter sequences that would allow the GFP reporter to indicate maximum PHB production.
Project Objectives
DESIGN PHB-reporter constructs (promoter region, reporter region, and PhaCAB cassette)
BUILD PHB-reporter constructs
TEST for functionality of constructs
Introduction
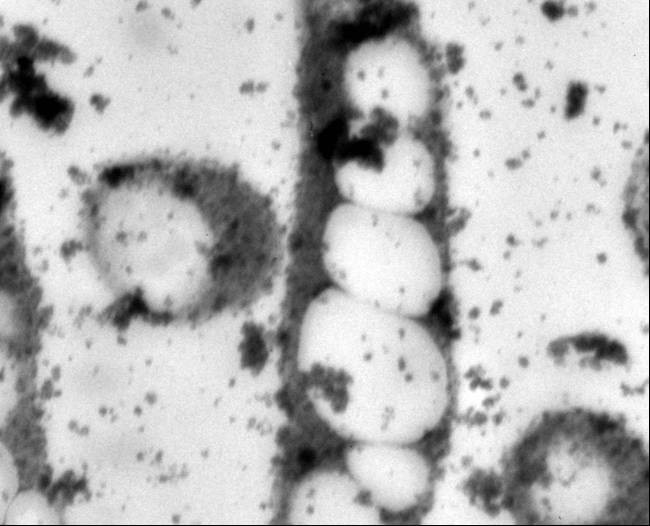
Polyhydroxybutyrate
Polyhydroxyalkanoates are a class of over 80 polyesters synthesized by more than 300 microorganisms that exhibit material properties similar to those of petrochemical plastics (Lee, 1999; Lee, 1996). Polyhydroxybutyrate (PHB), the most prevalent PHA, has been of particular interest in commercial and medical applications because of its high tensile strength, biodegradability, and biocompatibility with human tissues (Lee, 1996b; Abe, 1992; Williams 2002). Concerns with the increasingly limited supply of fossil fuels and public anxiety over landfills oversaturated with non-biodegradable materials have also stimulated bioplastic research (Moran, 2006; Ojumu, 2004). PHA research has increased in an effort to understand its potential. It has been used for specialized medical applications, including bone fixation, drug delivery systems and degradable sutures (Knowles, 1993: Kose, 2004; Holmes, 1985: Chen, 2005).
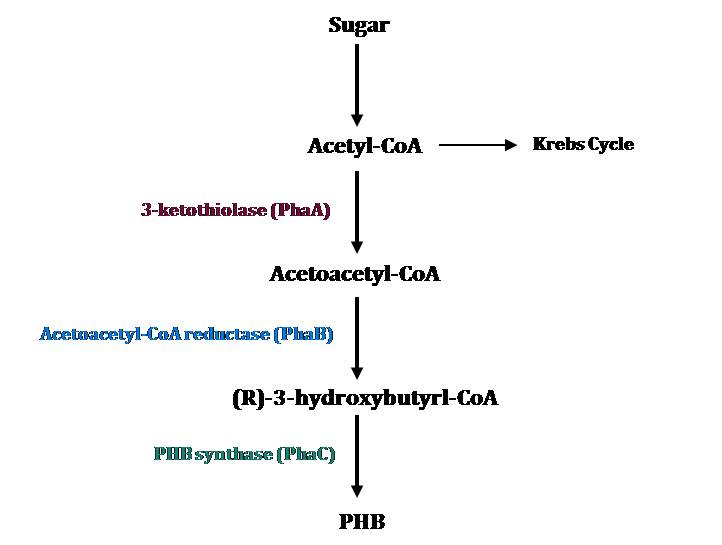
PHB Metabolic Pathways
The PHB cassette
Problems with PHB
1. COST
The current cost of industrial PHA production is estimated to be triple that of petrochemical plastics, which limits its widespread use.
2. PRODUCTION EFFICIENCY
Green Fluorescent Protein
GFP (Green Fluorescent Protein) is a protein originally isolated from the jellyfish Aequorea victoria, fluorescing when exposed to ultraviolet light. GFP is used as a tag, attached to proteins as a marker. Only those cells in which the tagged gene is expressed will fluoresce. In such a way GFP acts as a positive marker of tranformation. In our laboratory, GFP is used as an expression reporter of PHB. The GFP gene primarily used in this project was GFP E0240 BioBrick supplied by iGEM.
Methods
Organisms
Two organisms were used as genetic sources for PHB: Cupriavidus necator and an Escherichia coli strain containing the PHB operon in a pBluescript vector. The E. coli strain proved to be easier to use as a PCR template because of the ability to miniprep the plasmid DNA to use as more pure template.
Gel Electrophoresis
Polymerase Chain Reaction
Nine sequences were targeted out of the PHB operon for amplification. Five PCR targets were promoter sequences: 5’phaCproF/ATG R, 5’phaCproF/SD R, 5’phaCproF/TC R, -35F/ATG R, and -35F/TC R. Four PCR targets were from phaCAB gene complex: phaC, phaA, phaB, and phaCAB. A variety of annealing temperatures and Mg++ concentrations were tried, and it was found that 60˚C annealing temperature and 12% (v/v) Mg++ concentration were optimal. Two sets of primers were used: those without the BioBrick prefix and suffix attached and those with them already attached. The use of the PCR conditions just described enabled the use of the latter, saving subsequent ligations from needing to be done. Such PCR reactions were successful with all of the promoter regions and the phaB gene.
GFP Correlation
BioBrick Production
Results
Conclusions
References
1. Doi Y, Kunioka M, Nakamura Y, Soga K. 1986. Nuclear magnetic resonance studies on poly(B-hydroxybutyrate) and a copolyester of B-hydroxybutyrate and B-hydroxyvalerate isolated from Alcaligenes eutrophus H16. Macromolecules. 19:2860-2864. 2. Endy D. 2005. Foundations for engineering biology. Nature. 438(7067):449-53. International Genetically Engineered Machines competition. 17 Oct 2008. 26 Jul 2008. <https://igem.org>. 3. Kang Z, Wang Q, and H Zhang. 2008. Construction of a stress-induced system in Escherichia coli for efficient polyhydroxyalkanoates production. Biotechnological Products and Process Engineering. 79:203-208. 4. Knight TF. 2003. Idempotent Vector Design for Standard Assembly of BioBricks. Tech. rep., MIT Synthetic Biology Working Group Technical Reports. Registry of Standard Biological Parts. 17 Oct 2008. 26 Jul 2008 <http://partsregistry.org/Main_Page>. 5. Shetty RP, Endy D, Knight Jr TF. 2008. Engineering BioBrick vectors from BioBrick parts. Journal of Biological Engineering. 2:5. 6. Verlindin RAJ, Hill DJ, Kenward MA, Williams CD, and I Radecka. 2007. Bacterial synthesis of biodegradable Polyhydroxyalkanoates. Journal of Applied Microbiology 102:1437–1449.
 "
"