|
_transfection and synthetic receptor activation
Introduction
The functionality of our parts was analyzed in three steps. First, we tested the transfection protocol and checked the utility of the transfection vector CMV construct for expressing proteins in eucaryotic cells. Second, we analyzed protein expression level and cellular localization. Third, cotransfections were performed to show that clustering of two receptors leads to an assembly of the intracellular split fluorophores/-enzymes resulting in a functional protein.
Methods
Transfection of 293T cells
One day before transfection cells were counted in the Neubauer chamber and 6*10^4 cells/cm² were seeded in 6 well plates. Approximately 1 hour before transfection cells were washed with 1xPBS and fresh DMEM medium was added. For transfection 2 µg of DNA were mixed with 25 µl CaCl2 and ddH2O was filled up to 250 µl. After an incubation on ice for 20 min 250 µl BBS (2x) were added. This mixture was given to the cells and after 4-12 hours cells were washed and fresh medium was added.
ONPG (o-Nitrophenyl-ß-D-galactopyranosid) Test
Transfection was performed with a lac Z gene using the transfection protocol described above. After 48h one part of the cells was harvested by washing them in PBS and scraping them off. Then the cells were centrifuged at 13000 rpm for 2 min and the PBS was replaced by 500µl lysis buffer (1x). Incubation took place at -80°C for 20 min. After thawing, the solution was vortexed, spun down and the supernatant was frozen at -20°C. The same procedure was done with the rest of the cells one day later (68h). Then 20 µl of each lysate was given to 130 µl reaction buffer (incl. ONPG) letting the mixture incubate for 1h at 37°C. Measurement was done using the ELISA-reader at 405 nm.
Results and discussion
Testing the transfection protocol
In order to check if the transfection protocol (Ca2+ precipitation) is suitable to transfect 293T cells a 'test'transfection with a lac Z gene (Test-vector from Katja Arndt's lab) was done and the β-galactosidase was detected with an ONPG test.
ONPG-assay:

Table 1_Transfection: Absorbance of o-Nitrophenol produced by the β-galactosidase
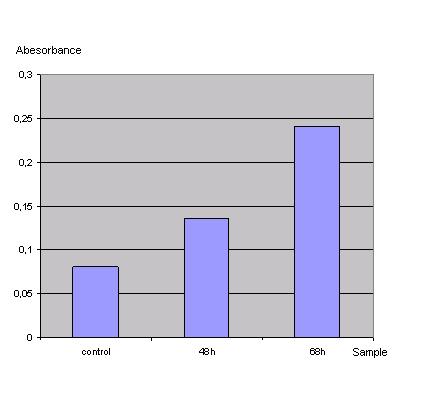
Graph 1_Transfection: Absorbance of o-Nitrophenol produced by the β-galactosidase (Control was done with untransfected cells using the same procedure)
Due to the results of the ONPG assay, the Ca2+ precipitation proved to be a very simple and effective method to transfect the 293T cells.
Testing the transfection vector CMV promoter construct
To test the functionality of the transfection vector and the CMV-promoter (BBa_K157040), YFP was cloned downstream of the promoter and the plasmid was brought into 293T cells. The detection of YFP took place 1 day later under a microscope with YFP filter.
The transfected cells show fluorescence by excitation of 510-520nm while the untransfected remain dark at this wavelength demonstrating the capability of the transfection vector CMV construct to induce protein expression in eucaryotic cells.
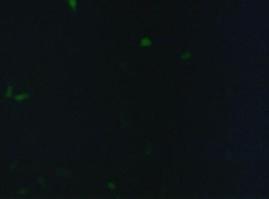  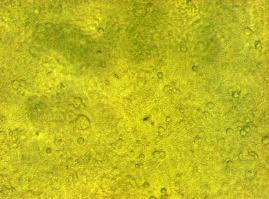
Figure 1_Transfection: Transfected 293T cells (left), untransfected cells (middle) and transfected cells transmitted light (right)
Localization at the cell membrane
To show the localization of the constructs at the cell membrane transfection of the construct signal_peptide-Lipocalin-transmembrane_region-betaLactamase1-YFP was performed.
Figure 2_Transfection shows the model of the protein structure based on PDB files. Anti-fluorescein Anticalin (BBa_K157004) is the extracellular part of the construct. The transmembrane region is identical to that of the EGF-receptor erbb1 (BBa_K157002). Split beta-Lactamase (BBa_I757011), the intracellular part is fused to the yellow fluorescent protein to detect membrane localization.

Figure 2_Transfection: Structure of the signalpeptide-Lipocalin-transmembraneregion-betaLactamase1-YFP construct. Extracellular: Lipocalin, GGGSlinker; Transmembrane: transmembraneregion of the EGF-receptor; Intracellular: Split-beta-Lactamase1, YFP.
Membrane localization of the construct signal_peptide-Lipocalin-transmembrane_region-betaLactamase1-YFP is visible in transfected 293T cells (Figure 3_Transfection). The fluorescence of the cells is most likely restricted to the cell membrane as evident by the strong signal at the surface of the cell spheres confirming the assembly of the construct in the cytoplasma membrane.
In comparison, 293T cells transfected with the construct transfection vector-CMV-YFP show a uniformly distributed fluorescence all over the cell (Figure 4_Transfection A and B).
Transfection with the construct transfection vector-CMV-CFP (BBa_K157041), i.e. without membrane targeting as well results in completely fluorescent cells (Figure 4_Transfection C and D).
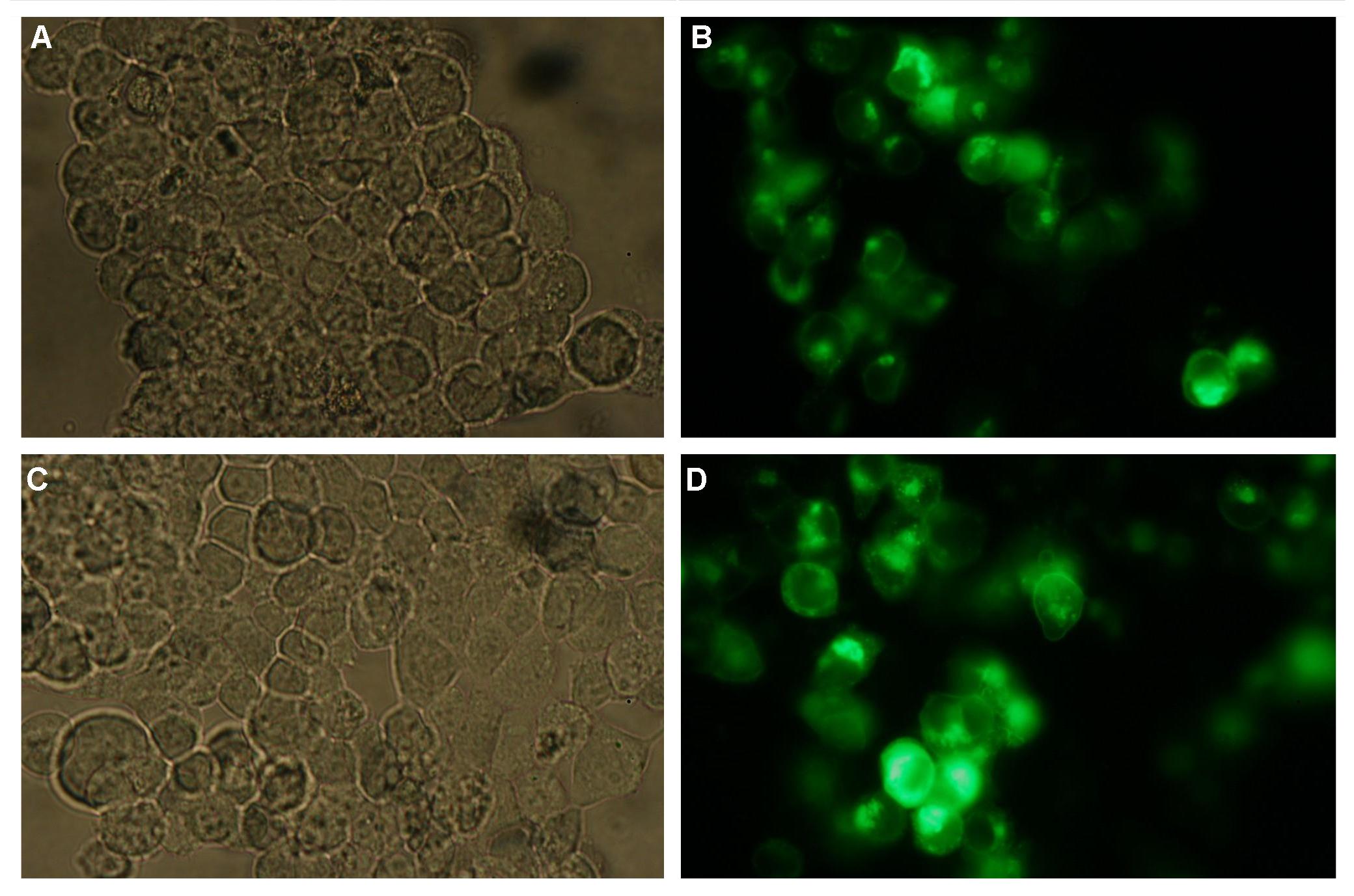
Figure 3_Transfection: 293T cells transfected with signal_peptide-Lipocalin-transmembrane_region-betaLactamase1-YFP.
This picture demonstrates the high expression level of the fusion protein and the localization at the cell membrane obvious by the strong fluorescent signal at the cell surface
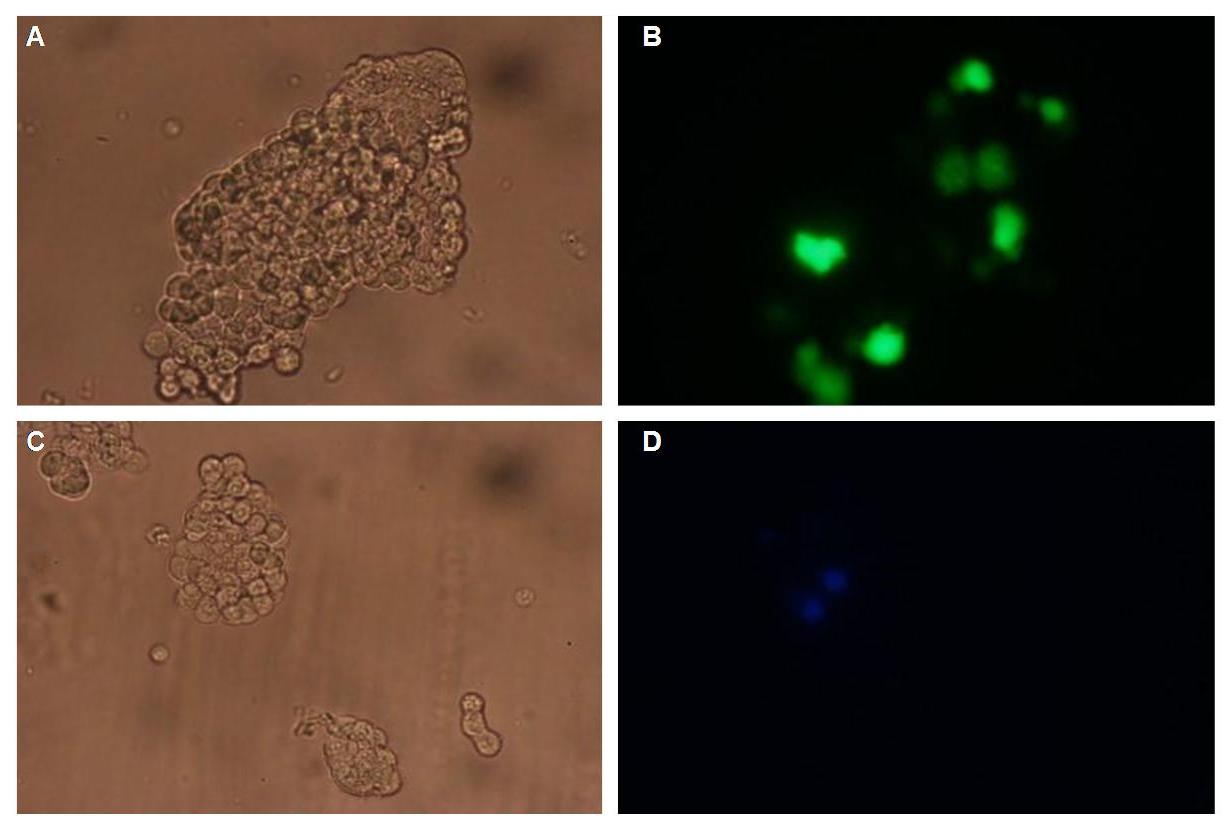
Figure 4_Transfection: 293T cells transfected with transfection vector-CMV-YFP (A and B); 293T cells transfected with transfection vector-CMV-CFP (C and D)
Fluorescence is uniformly distributed all over the cell and not restricted to the cell membrane
Cotransfections with Splitfluorophor-/Splitenzyme-constructs
Figure 5_Transfection gives the model of the Anticalin fused to the GGGSlinker, the transmembrane domain and the N-terminal part of Cerulean CFP or the C-terminal part of Cerulean CFP. To achieve more flexibility and to support the assembly of the two split fluorophore parts a fluolinker is fused in between the transmembrane region and the C-terminal part of the split fluorophores. The signal peptide is not displayed.

Figure 5_Transfection: Structures of the signal_peptide-Lipocalin-transmembrane_region-nCFP and signal_peptide-Lipocalin-transmembrane_region-fluolinker-cCFP constructs
We hypothesize that adding fluorescein-coupled molecules leads to a clustering of the Lipocalin constructs due to the fluorescein-Lipocalin-binding.
The clustering of the constructs in turn results in an assembly of the split fluorophores or split enzymes and therefore creates a functional protein (Figure 6_Transfection).

Figure 6_Transfection: Clustering and assembly of the signal_peptide-Lipocalin-transmembrane_region-nYFP and signal_peptide-Lipocalin-transmembrane_region-fluolinker-cYFP constructs
|