Home
People
Project Details
Protocols
Completed Systems
Biosafety
Support
|
Titering Bacteriophage
- Grow fresh overnight cultures of the bacteria of interest (i.e. LE392, D1210, etc.)
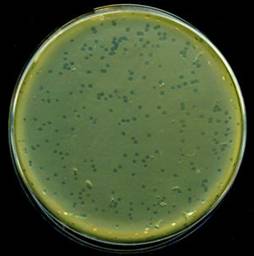 An example plate. Dark spots are cleared zones in a lawn of bacteria. - Incubate the cultures until they reach an OD600 of roughly 0.1
- More specifically, when the culture is swirled, cloudiness is observed
- Do NOT titer with bacterial cultures that have just been diluted from a culture past OD600 of 0.8 (complications will occur)
- Place agar/agarose plates in the 42 C oven and ready the 48 C water bath
- Prepare fresh 10 mM MgSO4 solution from the 1 M MgSO4 stock solution (100 ul MgSO4 in 10 mL sterile water works conveniently)
- In 1.5 mL eppendorf tubes, add 1 mL of the 10 mM MgSO4 solution.
- Prepare phage dilutions by adding appropriate amount of phage into MgSO4 solution (i.e. 1000-fold dilution is 1 ul phage in 1 ml MgSO4)
- After addition of phage to solution, invert tube at least 12 times for sufficient mixing
- For further dilutions, can simply dilute made phage dilutions (i.e. 1,000,000-fold can be obtained from taking 1 ul 1000-fold diluted phage and adding to another 1 mL of 10 mM MgSO4
- Aliquot 0.1 mL cell solution (i.e. LE392, D1210, etc.) into new 1.5 mL eppendorf tubes
- To begin infection, add appropriate amount of diluted phage to cell solution (10 ul usually works well for starters) and start the timer
- During this 30 minute infection period, label the plates and place back in the 42 C oven
- ~3 minutes before the end of infection, can take 3 mL aliquots of the top medium in 13 mL falcon tubes or 5 mL round-bottom tubes and microwave till complete liquification has occurred.
- Note that top agar is the same composition as is used to pour the plates, only with half the agar (so ~7g/L agar rather than 15 g/L).
- Right before the end of infection, take out the plates and place on the bench with the cover on top. Have a flame close-by to prevent contamination. Also, set the pippette to the right volume of infected cell solution and have sterile pipette non-filter tips ready
- Very quickly with pipette in hand, pippette out infected cell solution, add to top medium, invert the tube at least 2 times, and pour the mixed contents onto the corresponding plate.
- Rock the plate gently to allow the top medium to uniformly cover the plate and use the sterile tips to poke any bubbles.
- Let the plate cool on the bench for at least 5 minutes (10 minutes is usually enough) with the cover completely on the plate.
- Place the plate in the 37 C incubator.
  |