Imperial College/9 September 2008
From 2008.igem.org
8 September 2008
Wet Lab
- Taq PCR trials of genomic sequences set up and results analysed on gel
- Ligations of all available sequences into biobricks set up
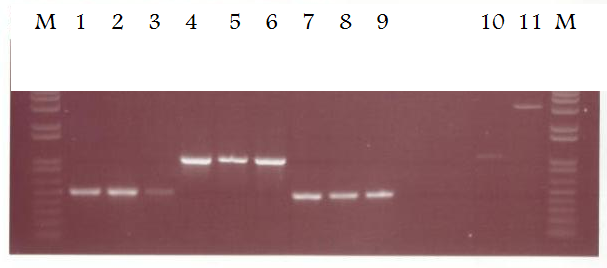
Lane 1 - EpsE 5' IS primers, chromosome template, oC for 10 cycles then 64oC for 20 cycles
Lane 2 - EpsE 5' IS primers, chromosome template, oC for 10 cycles then 64oC for 20 cycles
Lane 3 - EpsE 5' IS primers, chromosome template, oC for 10 cycles then 64oC for 20 cycles
Lane 4 - XylR primers, chromosome template, oC for 10 cycles then 60oC for 20 cycles
Lane 5 - XylR primers, chromosome template, oC for 10 cycles then 60oC for 20 cycles
Lane 6 - XylR primers, chromosome template, oC for 10 cycles then 60oC for 20 cycles
Lane 7 - EpsE 3' IS primers, chromosome template, oC for 10 cycles then 64oC for 20 cycles
Lane 8 - EpsE 3' IS primers, chromosome template, oC for 10 cycles then 64oC for 20 cycles
Lane 9 - EpsE 3' IS primers, chromosome template, oC for 10 cycles then 64oC for 20 cycles
Lane 10 - LacI insert (cut with XbaI and SpeI)
Lane 11 - pSB1AK3 vector (cut with XbaI and SpeI)
Conditions and timings were kept the same as the standard Taq protocol. Pfu PCR reactions using the optimal condiitons can now be set up to produce clones for use in our constructs. From the LacI and pSB1AK3 digests, it is now possible to decide how much of each is required in our ligation reaction
 "
"