Home
People
Project Details
Protocols
Completed Systems
Biosafety
Support
|
Subprojects
Note: Click on the subproject title or picture for a detailed description of the subproject
|
|
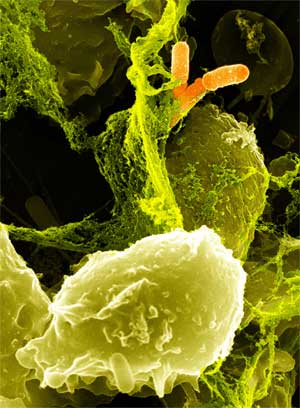
Specialized white blood cells called neutrophils defend us from illness by killing bacteria with a potent concoction of degradative enzymes and oxidizing agents, including hydrogen peroxide. However, pathogens of the human large intestine are able to cause serious illness while being sheltered from neutrophils. We engineered a strain of Escherichia coli that is able to mimic a neutrophil by producing cytotoxic amounts of hydrogen peroxide in a controlled, inducible manner. Our engineered E. coli use the transcriptional activator LuxR to detect the presence of acyl-homoserine lactones, quorum sensing signaling molecules secreted by invading pathogens. LuxR activates production of the pyruvate oxidase of Streptococcus pneumoniae, which produces large amounts of hydrogen peroxide by oxidizing pyruvate. The engineered E. coli is capable of killing certain strains of antibiotic resistant E. coli within six hours. When translated into a probiotic strain such as Nissle 1917, this system has the potential to be an effective means of combating enteric pathogens.
|
|
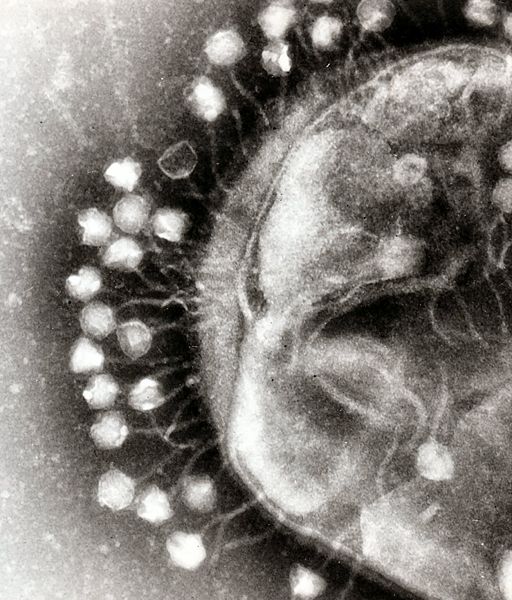
Another aspect of bacterial pathogen defense for our probiotic is to produce bacteriophages, which would rapidly infect and wipe out all of the pathogens. There are basically two methods to approach phage production, differentiated by the type of phage used. The first uses the bacteriophage λ, which targets E. Coli. The other is exploring the use of a temperate bacteriophage from B. Subtilis, however this method, if successful, can be adapted to temperate bacteriophages of any bacterial strain.
Bacteriophage λ is a temperate phage with an E. Coli. host, λ infects E. Coli through the lamB receptor, and absence of this receptor prevents λ infection. We will takes advantage of this aspect of bacteriophage λ to create E. Coli which are resistant to the phage, but release the phage to destroy susceptible pathogenic E. Coli.
The second approach is more versatile, and can target more strains of pathogenic bacteria. The goal is to create a phasmid out of the genome of a temperate bacteriophage. A phasmid combines a E. Coli plasmid Origin of Replication with a linear phage genome, circularizing it. This allows the phasmid to pass on as a plasmid within E. Coli, however when transferred to its native host, the phage phage is induced.
|
|
|
|
Approximately 75% of adults worldwide suffer from lactose intolerance, the inability to metabolize lactose in the small intestine. We propose to treat lactose intolerance by engineering a strain of Escherichia coli that can reside in the large intestine. The engineered strain will sense lactose and subsequently release ß-galactosidase to convert lactose into glucose and galactose, both of which can be reabsorbed by the host. To treat lactose intolerance, our engineered bacterial strain will contain two plasmids: one with constitutive expression of a mutant lactose permease and ß-galactosidase, and the second with lactose-inducible expression of the λ phage lysis cassette. The mutant lactose permease allows the cells to import lactose under all conditions. When the cells uptake enough lactose, the second plasmid will induce cell lysis through activation of the λ phage lysis cassette, resulting in cell lysis and release of ß-galactosidase into the large intestine. Data covering the construction and characterization of these plasmid constructs is discussed.
|
|
Folate, a term which encompasses the various forms of the vitamin B9, is an essential vitamin involved in everyday cell functions such as DNA replication. Unable to naturally produce folate, humans must obtain it from vegetables or folate-supplements. In regions with little or no access to these foods, folate deficiencies can cause serious birth defects. One possible solution to alleviate the effects of folate deficiency is to engineer a strain of gut microbes to produce bioavailable folate directly in the colon. A total of four heterologous genes, two from the folate biosynthesis gene cluster and two from the paraaminobenzoic acid (pABA) synthesis pathway, were tested. Using standardized genetic sequences, folate biosynthesis genes extracted from the Lactoccocus lactis genome were cloned into Biobricks plasmids, transformed into Escherichia coli and overexpressed. The effects of overexpression
were measured in terms of total folate and paraaminobenzoic acid levels. PABA, an intermediate in folate synthesis, was detected using high performance liquid chromatography (HPLC). Folate detection was achieved via a microbiological assay. A measurable increase in folate production in E. coli would be a proof-of-concept for both the feasibility of engineering overproduction of folate in E. coli as well as using standardized genetic components to do so.
|
|
|
|
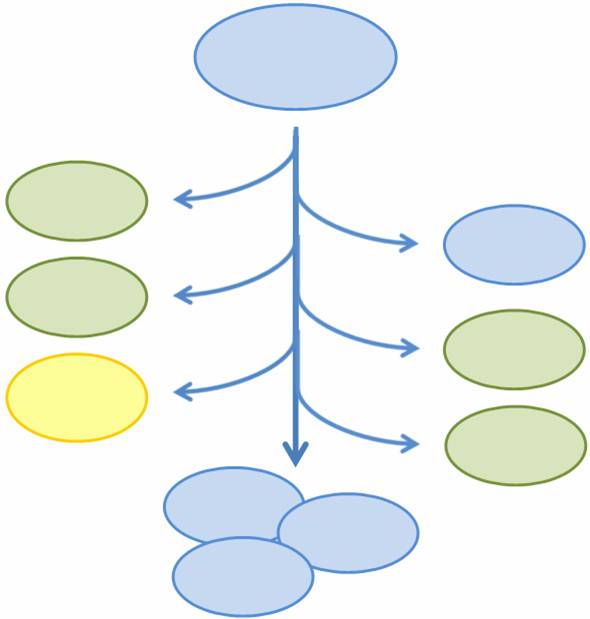
As more complicated and interconnected biological circuits are synthesized, there becomes the need for the simple integration of multiple functions into a single bacterial cell line. However, some of these functions may be incompatible or may kill the cell, such that each cell can only express a single function at any time and must be regenerated if the functionality is fatal. We aim to combine multiple mutually exclusive and potentially fatal functions into a single bacterial cell line that, as a population, expresses the entire set of functions.
In particular, we want only one of the four subprojects described above to be turned on in any given cell, or else the cell may be overburdened by our constructs. At the same time, we want our bacterial population to have all four abilities. In addition, each of the four subprojects causes the death of the host cell through self-induced lysis. We need a system that is able to combine all subprojects into one coherent system.
We propose a system in which bacterial cells initially start in an undifferentiated state and randomly differentiate into one of three possible final states. To build this system, we designed two genetic devices. One relies on DNA polymerase slippage upon replication of a long stretch of short nucleotide repeats, a phenomenon termed slipped-strand mutation (SSM). The other device uses the recombinase protein FimE to flip DNA segments. The two devices can form a system that permits the novel introduction of random multi-state differentiation into bacterial cells. Here, we present evidence that short nucleotide repeats can be used as a stochastic switch and that FimE activity depends on the length of the segment being flipped.
|
  |
 "
"