Team:Cambridge
From 2008.igem.org
(Difference between revisions)
| (68 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Cambridge/Notitle}} | ||
| + | <div class=bodytable> | ||
| + | </div> | ||
| + | __NOTOC__ | ||
| + | {{Cambridge08}} | ||
<html> | <html> | ||
| - | <style | + | <table width=100% style="background:#444; padding:15px;"> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | <tr> | |
| - | + | <td> | |
| - | + | <a href="https://2008.igem.org/Team:Cambridge/Signalling" class="noborder"><img src="http://openwetware.org/images/9/9d/Signalling_button.gif" alt="Signalling" width="250" style="padding: 3px 0px;"></a> | |
| - | + | <a href="https://2008.igem.org/Team:Cambridge/Bacillus"><img src="http://openwetware.org/images/8/8e/Bacillus_button.gif" alt="Bacillus" width="250" style="padding: 3px 0px;"></a> | |
| - | + | </td><td> | |
| - | + | <a href="https://2008.igem.org/Team:Cambridge/Voltage"><img src="http://openwetware.org/images/7/74/Voltage_button.gif" alt="Voltage" width="250" style="padding: 3px;"></a> | |
| - | + | <a href="https://2008.igem.org/Team:Cambridge/Modelling"><img src="http://openwetware.org/images/9/91/Modelling_button.gif" alt="Modelling" width="250" style="padding: 3px 0px;"></a> | |
| - | + | </td> | |
| - | + | <td style="height: 400; padding-left: 15px;"> | |
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; background:#fff; line-height:170% padding: 3px 0px;"> | ||
| + | <h1>Overview</h1> | ||
| + | <b>Since the emergence of Synthetic Biology</b>, bacteria have been engineered to perform a wide variety of simple tasks. They can be made to express proteins, respond to their environment and communicate primitively with each other. Presently, a key goal for the field is to create a communicating, organised and differentiated population of bacteria that can be considered a multicellular organism, capable of performing even more complex tasks. The ultimate goal for this line of research would be to mimic a brain, the most complex structure in the universe. To realize this goal requires the development of systems for rapid, robust communication and self-organised differentiation. <br /> <b> Our project sets the foundation for future research in engineered multi-cellularity by pursuing electrical and peptide signalling, and cellular self-differentiation through spontaneous spatial patterning.</b> | ||
| + | </div></div> | ||
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan=3> | ||
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; padding: 5px; background:#fff;"> | ||
| + | <h1>Voltage</h1> | ||
| + | In order to simulate neural activity in bacteria, a mechanism resembling a synapse is necessary. At the synapse, neurotransmitter molecules are released from the presynaptic plasma membrane. The neurotransmitter diffuses through the synaptic cleft and binds to chemical receptor molecules on the membrane of the postsynaptic cell. These receptors cause ion channels to open so that ions rush out, changing the transmembrane potential. Attempting to mimic this in a prokaryotic system is particularly attractive as, in a more general sense, it provides an interface between chemical or biological and electrical systems. <br /><b> Using the amino acid glutamate as our 'neurotransmitter', we have successfully demonstrated a voltage response in bacterial cells. </b> <a href="https://2008.igem.org/Team:Cambridge/Voltage"> Read on...</a> | ||
| + | </div> | ||
| + | </div> | ||
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan=3> | ||
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; padding: 5px; background:#fff;"> | ||
| + | <h1>Signalling</h1> | ||
| + | Using peptide-based signalling systems from gram-positive bacteria, we have laid the foundations for a self-organising biological system, capable of expressing spatial patterns of GFP expression on a bacterial lawn. The focus of our investigation was on a simple two-component Reaction-Diffusion system, allowing for simple spatial 'patterning' of gene expression. The simplest of these patterns mimic the spots and stripes seen on animal coats. In 1952, Alan Turing famously described this Reaction-Diffusion system and suggested it as the basis for self-organization and pattern formation in biological systems. <br /><b> This is a first step in the direction of engineering multicellular behaviour. </b> <a href="https://2008.igem.org/Team:Cambridge/Signalling"> Read on...</a> | ||
| + | </div> | ||
| + | </div> | ||
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan=3> | ||
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; padding: 5px; background:#fff;"> | ||
| + | <h1>Bacillus</h1> | ||
| + | To build more complex cellular systems, new tools and techniques are required. We are generating standardized parts, tools, and techniques for the gram-positive chassis ''B. subtillis''. Easy to handle and transform, this bacterium offers many adantages to ''E. coli', including the ability to secrete proteins and integrate DNA into the chromosome. We have designed, built, and submitted gram-positive RBSes, promoters, and shuttle vectors.<br /> <b> As a part of this work we have confirmed single copy chromosomal insertion, demonstrated InFusion assembly, and characterized an improved GFP variant. </b> | ||
| + | <a href="https://2008.igem.org/Team:Cambridge/Bacillus"> Read on...</a> | ||
| - | + | </div> | |
| + | </div> | ||
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan=3> | ||
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; padding: 5px; background:#fff;"> | ||
| + | <h1>Modelling</h1> | ||
| + | We have introduced a model of the AGR quorum-sensing system of S.aureus to illustrate how a typical quorum-sensing system works. The model predicts theoretical values for biological parameters (such as the threshold cell density) that can be verified and we will be also be able to predict how the system behaves if we change a number crucial parameters. This can be extremely useful in informing design decisions when building a synthetic device. We have also expanded this model into a hypothetical setup with a second parallel agr-system. <br /><b> Using this parallel signals model, we investigate how to engineer a biological patterning system.</b> <a href="https://2008.igem.org/Team:Cambridge/Modelling">Read on...</a> | ||
| + | </div> | ||
| + | </div> | ||
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan=3> | ||
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; padding: 5px; background:#fff; line-height:170%" align="center"> | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td width="200"><a href="http://www.labtech.co.uk/" class="noborder"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/labtech.jpg" alt="labtech" width="131" height="17"></a></td> | ||
| + | <td width="200"><a href="http://www.clontech-europe.com/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/clontech.jpg" alt="Clontech" width="112" height="34"></a></td> | ||
| + | <td width ="200"><a href="http://www.expressys.com/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/expressys.jpg" alt="expressys" width="112" height="70"></a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a href="http://www.invitrogen.com/site/us/en/home.html"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/invirtogen.jpg" alt="invitrogen" width="160" height="25"></a></td> | ||
| + | <td><a href="http://www.medical-solutions.co.uk/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/geneservice.jpg" alt="geneservice" width="140" height="26"></a></td> | ||
| + | <td><a href="http://www.bioscience.co.uk/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/cambridgebioscience.jpg" alt="cambridge bioscience" width="157" height="42"></a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a href="http://www.zymoresearch.com/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/zymoresearch.jpg" alt="zymo research" width="135" height="45"></a></td> | ||
| + | <td><a href="http://www.vwr.com/index.htm"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/vwr.jpg" alt="VWR" width="140" height="47"></a></td> | ||
| + | <td><a href="http://www.microzone.co.uk/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/microzone.gif" alt="Microzone" width="131" height="44"></a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a href="http://www.finnzymes.com/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/finnzymes.jpg" alt="Finnzymes" width="131" height="43"></a></td> | ||
| + | <td><a href="http://www.fisher.co.uk/"><img src="http://www.gen.cam.ac.uk/Images/logos/iGEMsponsors/fisherscientific.jpg" alt="Fisher Scientific" width="132" height="33" /></a></td> | ||
| + | <td><a href="http://www.dna20.com/"><img src="http://openwetware.org/images/8/88/Dna20_logo.jpg" alt="DNA 2.0" width="132" height="66" /></a></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </div> | ||
| + | </div> | ||
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td> | ||
| - | + | </tr> | |
| - | + | </table> | |
| - | + | </html> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 02:53, 30 October 2008

|
|
OverviewSince the emergence of Synthetic Biology, bacteria have been engineered to perform a wide variety of simple tasks. They can be made to express proteins, respond to their environment and communicate primitively with each other. Presently, a key goal for the field is to create a communicating, organised and differentiated population of bacteria that can be considered a multicellular organism, capable of performing even more complex tasks. The ultimate goal for this line of research would be to mimic a brain, the most complex structure in the universe. To realize this goal requires the development of systems for rapid, robust communication and self-organised differentiation.Our project sets the foundation for future research in engineered multi-cellularity by pursuing electrical and peptide signalling, and cellular self-differentiation through spontaneous spatial patterning. |
VoltageIn order to simulate neural activity in bacteria, a mechanism resembling a synapse is necessary. At the synapse, neurotransmitter molecules are released from the presynaptic plasma membrane. The neurotransmitter diffuses through the synaptic cleft and binds to chemical receptor molecules on the membrane of the postsynaptic cell. These receptors cause ion channels to open so that ions rush out, changing the transmembrane potential. Attempting to mimic this in a prokaryotic system is particularly attractive as, in a more general sense, it provides an interface between chemical or biological and electrical systems.Using the amino acid glutamate as our 'neurotransmitter', we have successfully demonstrated a voltage response in bacterial cells. Read on... |
||
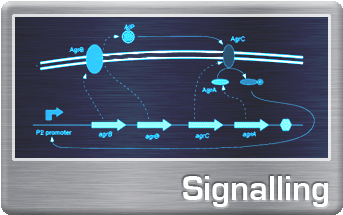
SignallingUsing peptide-based signalling systems from gram-positive bacteria, we have laid the foundations for a self-organising biological system, capable of expressing spatial patterns of GFP expression on a bacterial lawn. The focus of our investigation was on a simple two-component Reaction-Diffusion system, allowing for simple spatial 'patterning' of gene expression. The simplest of these patterns mimic the spots and stripes seen on animal coats. In 1952, Alan Turing famously described this Reaction-Diffusion system and suggested it as the basis for self-organization and pattern formation in biological systems.This is a first step in the direction of engineering multicellular behaviour. Read on... |
||
BacillusTo build more complex cellular systems, new tools and techniques are required. We are generating standardized parts, tools, and techniques for the gram-positive chassis ''B. subtillis''. Easy to handle and transform, this bacterium offers many adantages to ''E. coli', including the ability to secrete proteins and integrate DNA into the chromosome. We have designed, built, and submitted gram-positive RBSes, promoters, and shuttle vectors.As a part of this work we have confirmed single copy chromosomal insertion, demonstrated InFusion assembly, and characterized an improved GFP variant. Read on... |
||
ModellingWe have introduced a model of the AGR quorum-sensing system of S.aureus to illustrate how a typical quorum-sensing system works. The model predicts theoretical values for biological parameters (such as the threshold cell density) that can be verified and we will be also be able to predict how the system behaves if we change a number crucial parameters. This can be extremely useful in informing design decisions when building a synthetic device. We have also expanded this model into a hypothetical setup with a second parallel agr-system.Using this parallel signals model, we investigate how to engineer a biological patterning system. Read on... |
||
 "
"

