Team:Cambridge/Signalling/Lab Work
From 2008.igem.org
(→August, 16th) |
|||
| Line 10: | Line 10: | ||
For agrC we should have gotten a band of size 4300 (3000plasmid + 1300insert) | For agrC we should have gotten a band of size 4300 (3000plasmid + 1300insert) | ||
| - | |||
The bands of appropriate sizes were extracted for transformation. | The bands of appropriate sizes were extracted for transformation. | ||
| Line 30: | Line 29: | ||
=August, 19th= | =August, 19th= | ||
| + | ==Results of AgrA and C transformation== | ||
| + | Transformation has failed. No colonies were visible for the plates of Agr A or C. The Puc9 positive control grew. | ||
| + | We believe that the problem was in the gel-extraction between ligation and transformation. Most of our plasmid was probably lost in this step. Next time we will directly use the results from the ligation reaction to transform. Although many bands were seen on the previous gel of the ligation reaction, we will check our transformation growth by single colony PCR to confirm transformation of the plasmid with our correct insert. | ||
==Lux parts== | ==Lux parts== | ||
| Line 50: | Line 52: | ||
- Put on Kan plates 4 different colonies from J04630 (transformation Amp plate) and also incubate these colonies into LB | - Put on Kan plates 4 different colonies from J04630 (transformation Amp plate) and also incubate these colonies into LB | ||
| + | |||
| + | =August, 20th= | ||
| + | |||
| + | ==Check Lux components== | ||
| + | |||
| + | - Single colony PCR for : | ||
| + | |||
| + | *R0040 (MIT stuff) | ||
| + | |||
| + | *R0062 (MIT stuff) | ||
| + | |||
| + | *4 different colonies of S0168 (from a transformation plate from 12/08) | ||
| + | |||
| + | *4 different colonies of J04630 (from a transformation plate from 12/08) | ||
| + | |||
| + | - Protocol : add 1μL of cells (diluted in water), 10μL of Master Mis, 7μL of SDW, 1μL of VF primer and 1μL of VR primer | ||
| + | |||
| + | - Gel PCR products | ||
| + | |||
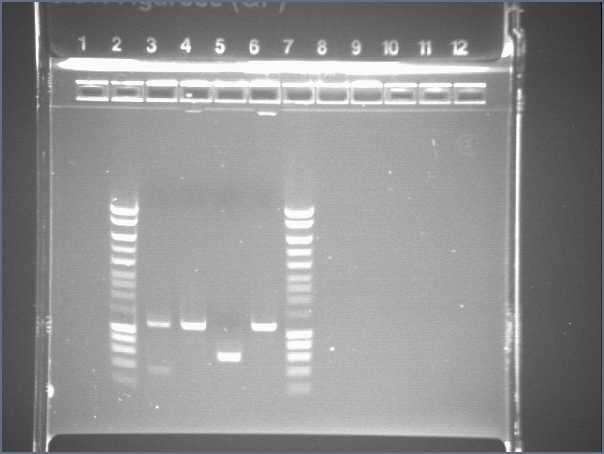
| + | Gel 1 | ||
| + | * Lane2 : Hyperladder1 | ||
| + | * Lane3 : JO4630, colony 1 | ||
| + | * Lane4 : JO4630, colony 2 | ||
| + | * Lane5 : JO4630, colony 3 | ||
| + | * Lane6 : JO4630, colony 4 | ||
| + | * Lane7 : HyperladderI | ||
| + | |||
| + | [[Image:photon.gif|300px|center]] | ||
| + | |||
| + | Gel 2 | ||
| + | |||
| + | * Lane2 : HyperladderI | ||
| + | * Lane3 : ECE190 double digest | ||
| + | * Lane4 : S0168, colony 1 | ||
| + | * Lane5 : S0168, colony 2 | ||
| + | * Lane6 : S0168, colony 3 | ||
| + | * Lane7 : S0168, colony 4 | ||
| + | * Lane8 : HyperladderI | ||
| + | * Lane9 : R0040 | ||
| + | * Lane10 : R0062 | ||
| + | * Lane11 : Ladder 100bp | ||
| + | |||
| + | [[Image:photoo.gif|300px|center]] | ||
| + | |||
| + | - '''Results''' | ||
| + | |||
| + | * R0040 and R0062 : one big band of about 300b (expected size 293), OK! | ||
| + | |||
| + | * S0168 : one band of about 400b for the 4 different colonies (expected size 1234!), bad! This plate does not contain S0168 | ||
| + | |||
| + | * J04630 (colonies 2 and 4) : one band of about 1100b (expected size 1173), OK! | ||
| + | |||
| + | * J04630 (colony 1) : one good band plus another band... | ||
| + | |||
| + | * J04630 (colony 3) : one band of about 600b, bad! | ||
| + | |||
| + | |||
| + | |||
| + | ==Ligation== | ||
| + | |||
| + | - Materials : | ||
| + | |||
| + | *AgrA | ||
| + | *AgrB | ||
| + | *AgrC | ||
| + | *AgrD | ||
| + | *Pupp | ||
| + | *Pspac | ||
| + | *Ppac | ||
| + | *Pxyl | ||
| + | *RBS S | ||
| + | *RBS W | ||
| + | *psB4C5 | ||
| + | |||
| + | - Double digest of PCR products | ||
| + | |||
| + | - Run vector, AgrA and AgrD on a gel | ||
| + | |||
| + | - DNA clean and concentrator for AgrA, B,C and D, promoters | ||
| + | |||
| + | - Microclean for both RBS | ||
| + | |||
| + | - Nanodrop | ||
| + | |||
| + | {|class="wikitable" style="text-align:center" border="1" | ||
| + | |- | ||
| + | ! !! 260/280 !! ng/μL | ||
| + | |- | ||
| + | | AgrA || 1.66 || 16.4 | ||
| + | |- | ||
| + | | AgrB || 1.91 || 23.5 | ||
| + | |- | ||
| + | | AgrC || 1.99 || 35.9 | ||
| + | |- | ||
| + | | AgrD || 2.13 || 4.9 | ||
| + | |- | ||
| + | | Pxyl || 1.54 || 5.6 | ||
| + | |- | ||
| + | | Ppac || 1.49 || 4.6 | ||
| + | |- | ||
| + | | Pspc || 1.62 || 9.6 | ||
| + | |- | ||
| + | | Pupp || 1.88 || 8.5 | ||
| + | |- | ||
| + | | RBS S || 2.44 || 29 | ||
| + | |- | ||
| + | | RBS W || 1.44 || 10.7 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | - Extract plasmid annd Agr from gel and clean | ||
| + | |||
| + | - Ligation | ||
| + | |||
<!-- ## Do not edit below this line unless you know what you are doing. ## --> | <!-- ## Do not edit below this line unless you know what you are doing. ## --> | ||
Revision as of 17:35, 28 October 2008
August, 16th
Agr A and C ligation to pSB4C5
Ligation was performed using Fermentas Rapid Ligation kit according to their protocol.
After ligation, samples were visualized on a gel, but little product of the correct size was seen.
For agrA we should have gotten a band of size 3700 (3000plasmid + 700 insert)
For agrC we should have gotten a band of size 4300 (3000plasmid + 1300insert)
The bands of appropriate sizes were extracted for transformation.
August, 18th
Check promoters
On Friday, PCR out promoters, we want to check them.
- Run a gel
- Results : good size of bands!!!
Transformation of AgrA and AgrC
AgrA and C gell-extracted ligation was transformed into Top 10 cells using standard protocol. Puc9 was used as a positive control.
August, 19th
Results of AgrA and C transformation
Transformation has failed. No colonies were visible for the plates of Agr A or C. The Puc9 positive control grew. We believe that the problem was in the gel-extraction between ligation and transformation. Most of our plasmid was probably lost in this step. Next time we will directly use the results from the ligation reaction to transform. Although many bands were seen on the previous gel of the ligation reaction, we will check our transformation growth by single colony PCR to confirm transformation of the plasmid with our correct insert.
Lux parts
To make the Lux Receiver, we need 4 different parts ;
- R0040, TetR repressible promoter
- SO168, luxR + double terminator
- R0062, promoter activated by luxR
- JO4630 (GFP + double terminator)
All these parts have been transformed into E.coli. We want to test them. R004, R0062 and JO4630 have already been tested, it should be fine. We received from the MIT R0040, R0062 and S068 already transformed into E.coli, so we want to check these stocks (which are certainly fine) and use them. For JO4630, we want to double check our transformation.
- Plate on antibiotic plates and do LB stocks of single colony from the MIT stock (R0040, R0062 and S0168).
- Put on Kan plates 4 different colonies from J04630 (transformation Amp plate) and also incubate these colonies into LB
August, 20th
Check Lux components
- Single colony PCR for :
- R0040 (MIT stuff)
- R0062 (MIT stuff)
- 4 different colonies of S0168 (from a transformation plate from 12/08)
- 4 different colonies of J04630 (from a transformation plate from 12/08)
- Protocol : add 1μL of cells (diluted in water), 10μL of Master Mis, 7μL of SDW, 1μL of VF primer and 1μL of VR primer
- Gel PCR products
Gel 1
- Lane2 : Hyperladder1
- Lane3 : JO4630, colony 1
- Lane4 : JO4630, colony 2
- Lane5 : JO4630, colony 3
- Lane6 : JO4630, colony 4
- Lane7 : HyperladderI
Gel 2
- Lane2 : HyperladderI
- Lane3 : ECE190 double digest
- Lane4 : S0168, colony 1
- Lane5 : S0168, colony 2
- Lane6 : S0168, colony 3
- Lane7 : S0168, colony 4
- Lane8 : HyperladderI
- Lane9 : R0040
- Lane10 : R0062
- Lane11 : Ladder 100bp
- Results
- R0040 and R0062 : one big band of about 300b (expected size 293), OK!
- S0168 : one band of about 400b for the 4 different colonies (expected size 1234!), bad! This plate does not contain S0168
- J04630 (colonies 2 and 4) : one band of about 1100b (expected size 1173), OK!
- J04630 (colony 1) : one good band plus another band...
- J04630 (colony 3) : one band of about 600b, bad!
Ligation
- Materials :
- AgrA
- AgrB
- AgrC
- AgrD
- Pupp
- Pspac
- Ppac
- Pxyl
- RBS S
- RBS W
- psB4C5
- Double digest of PCR products
- Run vector, AgrA and AgrD on a gel
- DNA clean and concentrator for AgrA, B,C and D, promoters
- Microclean for both RBS
- Nanodrop
| 260/280 | ng/μL | |
|---|---|---|
| AgrA | 1.66 | 16.4 |
| AgrB | 1.91 | 23.5 |
| AgrC | 1.99 | 35.9 |
| AgrD | 2.13 | 4.9 |
| Pxyl | 1.54 | 5.6 |
| Ppac | 1.49 | 4.6 |
| Pspc | 1.62 | 9.6 |
| Pupp | 1.88 | 8.5 |
| RBS S | 2.44 | 29 |
| RBS W | 1.44 | 10.7 |
- Extract plasmid annd Agr from gel and clean
- Ligation
 "
"