Team:Chiba/Calendar-Home/25 August 2008
From 2008.igem.org
(Difference between revisions)
(New page: '''OUTPUT TEAM''' <br>digestion <br>Lux1 <br>LUx2(1と2は別のコロニーをつついた) <br>Craig YFP <br>DNA 3μl <br>Buffer(x10) 1μl <br>dH2O 6μl <br>EcoRⅠ 0.5μl <b...) |
(→Team:Output) |
||
| (18 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | >[[Team:Chiba|Home]] | [[Team:Chiba/Notebook|Notebook]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | [[Team:Chiba/Calendar-Home/24 August 2008|24 August 2008 <]]|[[Team:Chiba/Calendar-Home/26 August 2008|> 26 August 2008]] |
| - | < | + | |
| - | < | + | ==Laboratory work== |
| - | < | + | ===Team:Input=== |
| - | < | + | The following glycerol Stock was made. |
| - | < | + | |
| - | < | + | [http://partsregistry.org/Part:BBa_J22136 BBa_J22136](insert check OK) |
| - | < | + | |
| - | < | + | <BR>Picked the colony and incubate with 2mL of LB-Ampicillin Medium for 12 hours at 37 degrees. |
| - | < | + | Took 180μl from culture,and mixed with 880μlLB-Amp Medium.(OD=0.485) |
| - | < | + | |
| - | < | + | <table width="210" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> |
| - | < | + | <td width="257">Liquid culture(OD=0.495)</td> |
| - | < | + | <td>0.67μl</td> |
| - | < | + | <tr> |
| - | < | + | <td>60%Glycerol</td> |
| - | < | + | <td>0.33μl</td> |
| + | </tr> | ||
| + | <tr> | ||
| + | <td>20%Glycerol Stock</td> | ||
| + | <td>1.00ml</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | ===Team:Communication=== | ||
| + | :(24/8)--->'''[[Team:Chiba/protocol/digestion|Digestion]]''' | ||
| + | ::#Double Digestion:[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) | ||
| + | ::#Double Digesiton:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) | ||
| + | ::#Single Digestion:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) | ||
| + | ::#[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) | ||
| + | |||
| + | <table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>1</td><td>2</td><td>3</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Sample DNA(μL)</td> | ||
| + | <td>5</td><td>5</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>EcoRI(μL)</td> | ||
| + | <td>0.5</td><td>0.5</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SpeI(μL)</td> | ||
| + | <td>0.5</td><td>0.5</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 1</td> | ||
| + | <td>1</td><td>1</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 3(μL)</td> | ||
| + | <td>-</td><td>-</td><td>1</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>BSA(μL)</td> | ||
| + | <td>1</td><td>1</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O(μL)</td> | ||
| + | <td>2</td><td>2</td><td>7.5</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL(μL)</td> | ||
| + | <td>10</td><td>10</td><td>10</td><td>1</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | :--->[[Team:Chiba/protocol/gelcheck|Gel Check]] | ||
| + | {|align="justify" | ||
| + | |[[Image:Chiba-0825-1.JPG]] | ||
| + | | | ||
| + | :<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>1~3</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Sample DNA(μL)</td> | ||
| + | <td>10</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Loading Dye(μL)</td> | ||
| + | <td>2</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O(μL)</td> | ||
| + | <td>-</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL(μL)</td> | ||
| + | <td>12</td><td>6</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | :From right; | ||
| + | ::#Double Digestion:[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) -> OK | ||
| + | ::#Double Digesiton:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) -> ?(low) | ||
| + | ::#Single Digestion:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) -> ?(low) | ||
| + | ::#[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) -> OK(low) | ||
| + | |} | ||
| + | |||
| + | |||
| + | --->[[Team:Chiba/protocol/digestion|Digestion]] | ||
| + | (Double Digestion) | ||
| + | ::#[http://partsregistry.org/Part:BBa_C0178 BBa_C0178] | ||
| + | ::#[http://partsregistry.org/Part:BBa_C0170 BBa_C0170] | ||
| + | ::#[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) | ||
| + | |||
| + | <table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>1</td><td>2</td><td>3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Sample DNA(μL)</td> | ||
| + | <td>33ng/μl×60μl</td><td>33ng/μl×60μl</td><td>100ng/μl×20μl</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PstI(μL)</td> | ||
| + | <td>2</td><td>2</td><td>2</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>XbaI(μL)</td> | ||
| + | <td>2</td><td>2</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SpeI(μL)</td> | ||
| + | <td>-</td><td>-</td><td>2</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 2(μL)</td> | ||
| + | <td>-</td><td>-</td><td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 3(μL)</td> | ||
| + | <td>10</td><td>10</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>BSA(μL)</td> | ||
| + | <td>10</td><td>10</td><td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O(μL)</td> | ||
| + | <td>16</td><td>16</td><td>16</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL(μL)</td> | ||
| + | <td>100</td><td>100</td><td>52</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | ===Team:Output=== | ||
| + | [[Team:Chiba/protocol/PCR|PCR]] | ||
| + | *[http://partsregistry.org/Part:BBa_E2030 BBa_E2030](1) | ||
| + | *[http://partsregistry.org/Part:BBa_J33202 BBa_J33202](2) | ||
| + | *BL21(3) | ||
| + | *[http://partsregistry.org/Part:BBa_J52008 BBa_J52008](4) | ||
| + | *[http://partsregistry.org/Part:BBa_I712019 BBa_I712019](5) | ||
| + | |||
| + | <table width="250" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">No.</td> | ||
| + | <td>1</td><td>2</td><td>3</td><td>4</td><td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA tamplate(μL)</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>FW primer(μL)</td> | ||
| + | <td>5(Venus_YFP_fwd)</td><td>5(LacZ_fwd)</td><td>5(LacZ_fwd)</td><td>5(rLUC_fwd)</td><td>5(fLuc_fwd)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>VR primer(μL)</td> | ||
| + | <td>5(VR)</td><td>5(LacZ_rev)</td><td>5(LacZ_rev)</td><td>5(VR)</td><td>5(VR)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dNTPmix(μL)</td> | ||
| + | <td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Thermo pol buffer(μL)</td> | ||
| + | <td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA pol(VENT)(μL)</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O(μL)</td> | ||
| + | <td>68</td><td>68</td><td>68</td><td>68</td><td>68</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL(μL)</td> | ||
| + | <td>100</td><td>100</td><td>100</td><td>100</td><td>100</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | -->[[Team:Chiba/protocol/gelcheck|Gel Check]] | ||
| + | |||
| + | |||
| + | <table width="250" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">No.</td> | ||
| + | <td>1</td><td>2</td><td>3</td><td>4</td><td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA tamplate(μL)</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>loading Dye(μL)</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O(μL)</td> | ||
| + | <td>4</td><td>4</td><td>4</td><td>4</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL(μL)</td> | ||
| + | <td>6</td><td>6</td><td>6</td><td>6</td><td>6</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | [[Team:Chiba/protocol/DNA_Purification/sigma|Mini prep]] | ||
| + | *[http://partsregistry.org/Part:BBa_E2030 BBa_E2030] | ||
| + | *[http://partsregistry.org/Part:BBa_F2620 BBa_F2620] | ||
| + | |||
| + | |||
| + | [[Team:Chiba/protocol/transformation|Transformation]] | ||
| + | *[http://partsregistry.org/Part:BBa_E2030 BBa_E2030] | ||
| + | *PUC19 | ||
| + | *pGFPUV | ||
| + | *pGEX Venus YFP | ||
| + | *Membren Venus YFP | ||
| + | [[Team:Chiba/protocol/PCR|PCR]] | ||
| + | *[http://partsregistry.org/Part:BBa_J63001 BBa_J63001](1) | ||
| + | <table width="250" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">No.</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA tamplate(μL)</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>FW primer(Venus)(μL)</td> | ||
| + | <td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>VR primer(μL)</td> | ||
| + | <td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dNTPmix(μL)</td> | ||
| + | <td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Thermo pol buffer(μL)</td> | ||
| + | <td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA pol(VENT)(μL)</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O(μL)</td> | ||
| + | <td>68</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL(μL)</td> | ||
| + | <td>100</td> | ||
| + | </tr> | ||
| + | </table> | ||
Latest revision as of 06:15, 30 October 2008
24 August 2008 <|> 26 August 2008
Contents |
Laboratory work
Team:Input
The following glycerol Stock was made.
BBa_J22136(insert check OK)
Picked the colony and incubate with 2mL of LB-Ampicillin Medium for 12 hours at 37 degrees.
Took 180μl from culture,and mixed with 880μlLB-Amp Medium.(OD=0.485)
| Liquid culture(OD=0.495) | 0.67μl |
| 60%Glycerol | 0.33μl |
| 20%Glycerol Stock | 1.00ml |
Team:Communication
- (24/8)--->Digestion
- Double Digestion:BBa_J04500(2007)
- Double Digesiton:BBa_R0010(2007)
- Single Digestion:BBa_R0010(2007)
- BBa_R0010(2007)
| Sample No. | 1 | 2 | 3 | 4 |
| Sample DNA(μL) | 5 | 5 | 1 | 1 |
| EcoRI(μL) | 0.5 | 0.5 | - | - |
| SpeI(μL) | 0.5 | 0.5 | - | - |
| Buffer 1 | 1 | 1 | - | - |
| Buffer 3(μL) | - | - | 1 | - |
| BSA(μL) | 1 | 1 | - | - |
| dH2O(μL) | 2 | 2 | 7.5 | - |
| TOTAL(μL) | 10 | 10 | 10 | 1 |
- --->Gel Check
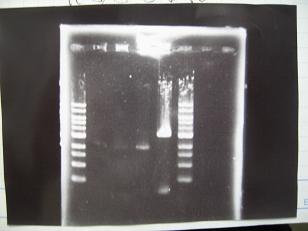
|
|
--->Digestion
(Double Digestion)
- BBa_C0178
- BBa_C0170
- BBa_J04500(2007)
| Sample No. | 1 | 2 | 3 |
| Sample DNA(μL) | 33ng/μl×60μl | 33ng/μl×60μl | 100ng/μl×20μl |
| PstI(μL) | 2 | 2 | 2 |
| XbaI(μL) | 2 | 2 | - |
| SpeI(μL) | - | - | 2 |
| Buffer 2(μL) | - | - | 5 |
| Buffer 3(μL) | 10 | 10 | - |
| BSA(μL) | 10 | 10 | 5 |
| dH2O(μL) | 16 | 16 | 16 |
| TOTAL(μL) | 100 | 100 | 52 |
Team:Output
- BBa_E2030(1)
- BBa_J33202(2)
- BL21(3)
- BBa_J52008(4)
- BBa_I712019(5)
| No. | 1 | 2 | 3 | 4 | 5 |
| DNA tamplate(μL) | 1 | 1 | 1 | 1 | 1 |
| FW primer(μL) | 5(Venus_YFP_fwd) | 5(LacZ_fwd) | 5(LacZ_fwd) | 5(rLUC_fwd) | 5(fLuc_fwd) |
| VR primer(μL) | 5(VR) | 5(LacZ_rev) | 5(LacZ_rev) | 5(VR) | 5(VR) |
| dNTPmix(μL) | 10 | 10 | 10 | 10 | 10 |
| Thermo pol buffer(μL) | 10 | 10 | 10 | 10 | 10 |
| DNA pol(VENT)(μL) | 1 | 1 | 1 | 1 | 1 |
| dH2O(μL) | 68 | 68 | 68 | 68 | 68 |
| TOTAL(μL) | 100 | 100 | 100 | 100 | 100 |
-->Gel Check
| No. | 1 | 2 | 3 | 4 | 5 |
| DNA tamplate(μL) | 1 | 1 | 1 | 1 | 1 |
| loading Dye(μL) | 1 | 1 | 1 | 1 | 1 |
| dH2O(μL) | 4 | 4 | 4 | 4 | 4 |
| TOTAL(μL) | 6 | 6 | 6 | 6 | 6 |
- BBa_E2030
- PUC19
- pGFPUV
- pGEX Venus YFP
- Membren Venus YFP
- BBa_J63001(1)
| No. | 1 |
| DNA tamplate(μL) | 1 |
| FW primer(Venus)(μL) | 5 |
| VR primer(μL) | 5 |
| dNTPmix(μL) | 10 |
| Thermo pol buffer(μL) | 10 |
| DNA pol(VENT)(μL) | 1 |
| dH2O(μL) | 68 |
| TOTAL(μL) | 100 |
 "
"