Team:Chiba/Calendar-Home/25 August 2008
From 2008.igem.org
(Difference between revisions)
(New page: '''OUTPUT TEAM''' <br>digestion <br>Lux1 <br>LUx2(1と2は別のコロニーをつついた) <br>Craig YFP <br>DNA 3μl <br>Buffer(x10) 1μl <br>dH2O 6μl <br>EcoRⅠ 0.5μl <b...) |
|||
| Line 1: | Line 1: | ||
| - | + | >[[Team:Chiba|Home]] | [[Team:Chiba/Notebook|Notebook]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | [[Team:Chiba/Calendar-Home/25 August 2008|25 August 2008 <]]|[[Team:Chiba/Calendar-Home/26 August 2008|> 26 August 2008]] |
| - | < | + | |
| - | < | + | ==Laboratory work== |
| - | < | + | ===Team:Input=== |
| - | < | + | [http://partsregistry.org/Part:BBa_J22136 BBa_J22136](insert check済み)のグリセロールストックづくり |
| - | < | + | <BR><BR>プレートに生えたコロニーをpickして、試験管で2ml培養(LB-Amp,37℃,12h) |
| - | < | + | <BR>培養した試験管から180μl,LB-Ampを880μlとって、OD=0.495にした。 |
| - | < | + | <BR><table width="210" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> |
| - | < | + | <td width="257">OD=0.495培養液</td> |
| - | < | + | <td>0.67μl</td> |
| - | < | + | <tr> |
| - | < | + | <td>60%グリセロール</td> |
| - | < | + | <td>0.33μl</td> |
| - | < | + | </tr> |
| - | < | + | <tr> |
| - | < | + | <td>20%グリセロールストック(計)</td> |
| - | < | + | <td>1.00ml</td> |
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | ===Team:Communication===:(24/8)--->'''[[Team:Chiba/protocol/digestion|Digestion]]''' | ||
| + | ::#Double Digestion:[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) | ||
| + | ::#Double Digesiton:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) | ||
| + | ::#Single Digestion:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) | ||
| + | ::#[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) | ||
| + | |||
| + | <table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>1</td><td>2</td><td>3</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Sample DNA</td> | ||
| + | <td>5</td><td>5</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>EcoRⅠ</td> | ||
| + | <td>0.5</td><td>0.5</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SpeⅠ</td> | ||
| + | <td>0.5</td><td>0.5</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 1</td> | ||
| + | <td>1</td><td>1</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 3</td> | ||
| + | <td>-</td><td>-</td><td>1</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>BSA</td> | ||
| + | <td>1</td><td>1</td><td>-</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>2</td><td>2</td><td>7.5</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>10</td><td>10</td><td>10</td><td>1</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | :--->[[Team:Chiba/protocol/gelcheck|Gel Check]] | ||
| + | {|align="justify" | ||
| + | |[[Image:Chiba-0825-1.JPG]] | ||
| + | | | ||
| + | :<table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>1~3</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Sample DNA</td> | ||
| + | <td>10</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Loading Dye</td> | ||
| + | <td>2</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>-</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>12</td><td>6</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | :From right; | ||
| + | ::#Double Digestion:[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) -> OK | ||
| + | ::#Double Digesiton:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) -> ?(low) | ||
| + | ::#Single Digestion:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) -> ?(low) | ||
| + | ::#[http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007) -> OK(low) | ||
| + | |} | ||
| + | |||
| + | |||
| + | --->[[Team:Chiba/protocol/digestion|Digestion]] | ||
| + | (Double Digestion) | ||
| + | ::#[http://partsregistry.org/Part:BBa_C0178 BBa_C0178] | ||
| + | ::#[http://partsregistry.org/Part:BBa_C0170 BBa_C0170] | ||
| + | ::#[http://partsregistry.org/Part:BBa_J04500 BBa_J04500](2007) | ||
| + | |||
| + | <table width="315" border="2" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>1</td><td>2</td><td>3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Sample DNA</td> | ||
| + | <td>33ng/μl×60μl</td><td>33ng/μl×60μl</td><td>100ng/μl×20μl</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PstⅠ</td> | ||
| + | <td>2</td><td>2</td><td>2</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>XbaⅠ</td> | ||
| + | <td>2</td><td>2</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SpeⅠ</td> | ||
| + | <td>-</td><td>-</td><td>2</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 2</td> | ||
| + | <td>-</td><td>-</td><td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Buffer 3</td> | ||
| + | <td>10</td><td>10</td><td>-</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>BSA</td> | ||
| + | <td>10</td><td>10</td><td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>16</td><td>16</td><td>16</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>100</td><td>100</td><td>52</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | |||
| + | ===Team:Output===[[Team:Chiba/protocol/PCR|PCR]] | ||
| + | *[http://partsregistry.org/Part:BBa_E2030 BBa_E2030]① | ||
| + | *[http://partsregistry.org/Part:BBa_J33202 BBa_J33202]② | ||
| + | *BL21③ | ||
| + | *[http://partsregistry.org/Part:BBa_J52008 BBa_J52008]④ | ||
| + | *[http://partsregistry.org/Part:BBa_I712019 BBa_I712019]⑤ | ||
| + | |||
| + | <table width="250" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>①</td><td>②</td><td>③</td><td>④</td><td>⑤</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA tamplate</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td>><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>FW primer</td> | ||
| + | <td>5(Venus_YFP_fwd)</td><td>5(LacZ_fwd)</td><td>5(LacZ_fwd)</td><td>5(rLUC_fwd)</td><td>5(fLuc_fwd)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>VR primer</td> | ||
| + | <td>5(VR)</td><td>5(LacZ_rev)</td><td>5(LacZ_rev)</td><td>5(VR)</td><td>5(VR)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dNTPmix</td> | ||
| + | <td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Thermo pol buffer</td> | ||
| + | <td>10</td><td>10</td><td>10</td><td>10</td><td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA pol(VENT)</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>68</td><td>68</td><td>68</td><td>68</td><td>68</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>100</td><td>100</td><td>100</td><td>100</td><td>100</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | -->[[Team:Chiba/protocol/gelcheck|Gel Check]] | ||
| + | |||
| + | |||
| + | <table width="250" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>①</td><td>②</td><td>③</td><td>④</td><td>⑤</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA tamplate</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td>><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>loading Dye</td> | ||
| + | <td>1</td><td>1</td><td>1</td><td>1</td><td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>4</td><td>4</td><td>4</td><td>4</td><td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>6</td><td>6</td><td>6</td><td>6</td><td>6</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | [[Team:Chiba/protocol/DNA_Purification/sigma|Mini prep]] | ||
| + | *Venus | ||
| + | *[http://partsregistry.org/Part:BBa_F2620 BBa_F2620] | ||
| + | |||
| + | |||
| + | [[Team:Chiba/protocol/transformation|Transformation]] | ||
| + | *Venus | ||
| + | *PUC19 | ||
| + | *pGFPUV | ||
| + | *pGEX Venus YFP | ||
| + | *Membren Venus YFP | ||
| + | [[Team:Chiba/protocol/PCR|PCR]] | ||
| + | *[http://partsregistry.org/Part:BBa_J63001 BBa_J63001]① | ||
| + | <table width="250" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
| + | <tr> | ||
| + | <td width="257">Sample No.</td> | ||
| + | <td>①</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA tamplate</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>FW primer(Venus)</td> | ||
| + | <td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>VR primer</td> | ||
| + | <td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dNTPmix</td> | ||
| + | <td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Thermo pol buffer</td> | ||
| + | <td>10</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DNA pol(VENT)</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>dH<sub>2</sub>O</td> | ||
| + | <td>68</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>TOTAL</td> | ||
| + | <td>100</td> | ||
| + | </tr> | ||
| + | </table> | ||
Revision as of 07:05, 24 October 2008
25 August 2008 <|> 26 August 2008
Laboratory work
Team:Input
BBa_J22136(insert check済み)のグリセロールストックづくり
プレートに生えたコロニーをpickして、試験管で2ml培養(LB-Amp,37℃,12h)
培養した試験管から180μl,LB-Ampを880μlとって、OD=0.495にした。
| OD=0.495培養液 | 0.67μl |
| 60%グリセロール | 0.33μl |
| 20%グリセロールストック(計) | 1.00ml |
===Team:Communication===:(24/8)--->Digestion
- Double Digestion:BBa_J04500(2007)
- Double Digesiton:BBa_R0010(2007)
- Single Digestion:BBa_R0010(2007)
- BBa_R0010(2007)
| Sample No. | 1 | 2 | 3 | 4 |
| Sample DNA | 5 | 5 | 1 | 1 |
| EcoRⅠ | 0.5 | 0.5 | - | - |
| SpeⅠ | 0.5 | 0.5 | - | - |
| Buffer 1 | 1 | 1 | - | - |
| Buffer 3 | - | - | 1 | - |
| BSA | 1 | 1 | - | - |
| dH2O | 2 | 2 | 7.5 | - |
| TOTAL | 10 | 10 | 10 | 1 |
- --->Gel Check
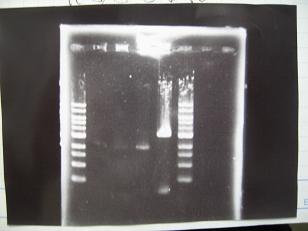
|
|
--->Digestion
(Double Digestion)
- BBa_C0178
- BBa_C0170
- BBa_J04500(2007)
| Sample No. | 1 | 2 | 3 |
| Sample DNA | 33ng/μl×60μl | 33ng/μl×60μl | 100ng/μl×20μl |
| PstⅠ | 2 | 2 | 2 |
| XbaⅠ | 2 | 2 | - |
| SpeⅠ | - | - | 2 |
| Buffer 2 | - | - | 5 |
| Buffer 3 | 10 | 10 | - |
| BSA | 10 | 10 | 5 |
| dH2O | 16 | 16 | 16 |
| TOTAL | 100 | 100 | 52 |
===Team:Output===PCR
- BBa_E2030①
- BBa_J33202②
- BL21③
- BBa_J52008④
- BBa_I712019⑤
| Sample No. | ① | ② | ③ | ④ | ⑤ |
| DNA tamplate | 1 | 1 | 1 | 1 | >1 |
| FW primer | 5(Venus_YFP_fwd) | 5(LacZ_fwd) | 5(LacZ_fwd) | 5(rLUC_fwd) | 5(fLuc_fwd) |
| VR primer | 5(VR) | 5(LacZ_rev) | 5(LacZ_rev) | 5(VR) | 5(VR) |
| dNTPmix | 10 | 10 | 10 | 10 | 10 |
| Thermo pol buffer | 10 | 10 | 10 | 10 | 10 |
| DNA pol(VENT) | 1 | 1 | 1 | 1 | 1 |
| dH2O | 68 | 68 | 68 | 68 | 68 |
| TOTAL | 100 | 100 | 100 | 100 | 100 |
-->Gel Check
| Sample No. | ① | ② | ③ | ④ | ⑤ |
| DNA tamplate | 1 | 1 | 1 | 1 | >1 |
| loading Dye | 1 | 1 | 1 | 1 | 1 |
| dH2O | 4 | 4 | 4 | 4 | 4 |
| TOTAL | 6 | 6 | 6 | 6 | 6 |
- Venus
- BBa_F2620
- Venus
- PUC19
- pGFPUV
- pGEX Venus YFP
- Membren Venus YFP
| Sample No. | ① |
| DNA tamplate | 1 |
| FW primer(Venus) | 5 |
| VR primer | 5 |
| dNTPmix | 10 |
| Thermo pol buffer | 10 |
| DNA pol(VENT) | 1 |
| dH2O | 68 |
| TOTAL | 100 |
 "
"